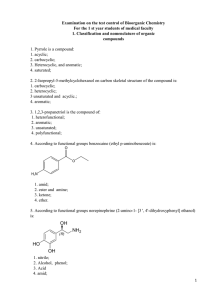
tro2_ppt_lecture_04 - Louisiana Tech University
... • If all 5.40 g Al were used, then 10.2 g of Al2O3 would be produced. 10.2 g < 17.2 g • The limiting reactant is Al. • Theoretical yield is 10.2 g Al2O3. To determine the percent yield of the reaction: (4.50 g/10.2) x 100 = 44.1% 44.1% is the percent yield for this reaction. © 2013 Pearson Education ...
... • If all 5.40 g Al were used, then 10.2 g of Al2O3 would be produced. 10.2 g < 17.2 g • The limiting reactant is Al. • Theoretical yield is 10.2 g Al2O3. To determine the percent yield of the reaction: (4.50 g/10.2) x 100 = 44.1% 44.1% is the percent yield for this reaction. © 2013 Pearson Education ...
Chapter 5
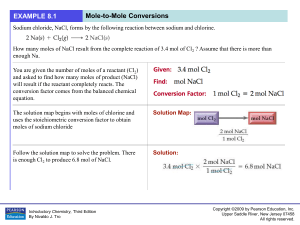
... H2SO4 are needed to react completely with 25.0 mL of 0.400 M NaOH? 2 NaOH(aq) + H2SO4(aq) -----> Na2SO4(aq) + 2 H2O (25.0 mL NaOH) (0.400 mol NaOH) (1 L) (1 mol H2SO4) --------------------------------#mL H2SO4 = ----------------------------------------------(1 L NaOH) (1000 mL)(2 mol NaOH) ...
... H2SO4 are needed to react completely with 25.0 mL of 0.400 M NaOH? 2 NaOH(aq) + H2SO4(aq) -----> Na2SO4(aq) + 2 H2O (25.0 mL NaOH) (0.400 mol NaOH) (1 L) (1 mol H2SO4) --------------------------------#mL H2SO4 = ----------------------------------------------(1 L NaOH) (1000 mL)(2 mol NaOH) ...
chemistry - Ethiopian Ministry of Education
... 1.1.2 Major Fields of Chemistry The universe is just like a very big chemical laboratory, rearranging atoms and subatomic particles to produce elements and compounds. While planets are made up of rocks which are nothing but arrangement of compounds, an atmosphere is a mixture of compounds separated ...
... 1.1.2 Major Fields of Chemistry The universe is just like a very big chemical laboratory, rearranging atoms and subatomic particles to produce elements and compounds. While planets are made up of rocks which are nothing but arrangement of compounds, an atmosphere is a mixture of compounds separated ...
Name:
... According to these results, what would be the initial rate (in mol/(L·s)) if all three concentrations are: [BrO3-]=[Br-]=[H+]=0.20 mol/L? 2. Use the following diagram to answer the questions below. a) Is the reaction exothermic or endothermic? Explain. b) What letter represents the activation energy ...
... According to these results, what would be the initial rate (in mol/(L·s)) if all three concentrations are: [BrO3-]=[Br-]=[H+]=0.20 mol/L? 2. Use the following diagram to answer the questions below. a) Is the reaction exothermic or endothermic? Explain. b) What letter represents the activation energy ...
CfE Advanced Higher Chemistry
... up as dark lines on a continuous spectrum and is called an atomic absorption spectrum, see Figure 1.4 (c). This also provides a pattern that can often be used in identification. In both techniques some lines normally occur in the visible region (400-700 nm) but some applications use the ultraviolet ...
... up as dark lines on a continuous spectrum and is called an atomic absorption spectrum, see Figure 1.4 (c). This also provides a pattern that can often be used in identification. In both techniques some lines normally occur in the visible region (400-700 nm) but some applications use the ultraviolet ...
Reduction of CuO in H2: in situ time
... CuO, Cu4 O3 and Cu2 O are oxides of copper with welldefined crystal structures [15–17]. Thus, a sequential reduction of copper oxide (CuO ) Cu4 O3 ) Cu2 O ) Cu) upon reaction with H2 could occur. The data reported in the literature do not agree on this point. Experiments of H2 temperature-programmed ...
... CuO, Cu4 O3 and Cu2 O are oxides of copper with welldefined crystal structures [15–17]. Thus, a sequential reduction of copper oxide (CuO ) Cu4 O3 ) Cu2 O ) Cu) upon reaction with H2 could occur. The data reported in the literature do not agree on this point. Experiments of H2 temperature-programmed ...
Chap 3 - HCC Learning Web
... “Balance the equation when butane gas reacts with oxygen gas (combustion or burning)” or “write a balanced chemical equation when butane reacts with oxygen”. Balancing equation is a very, very important question. To balance a chemical equation, you must make sure the number of atoms of each kind at ...
... “Balance the equation when butane gas reacts with oxygen gas (combustion or burning)” or “write a balanced chemical equation when butane reacts with oxygen”. Balancing equation is a very, very important question. To balance a chemical equation, you must make sure the number of atoms of each kind at ...
Chapter 5
... The energy required to obtain these complexes is not low, but as we will describe later, the overall reaction is highly exothermic and the reaction path based on these π-allyl complexes satisfactory justify the products obtained and also their distribution. On the other hand, the experimental condit ...
... The energy required to obtain these complexes is not low, but as we will describe later, the overall reaction is highly exothermic and the reaction path based on these π-allyl complexes satisfactory justify the products obtained and also their distribution. On the other hand, the experimental condit ...
Exam 2 Key
... 3. The terms strong electrolyte and weak electrolyte are used in multiple contexts. Discuss how these terms are used in each of the contexts below. Use a maximum of three sentences per context. (8 points) a. When describing a compound: When describing a compound, the term electrolyte refers to the c ...
... 3. The terms strong electrolyte and weak electrolyte are used in multiple contexts. Discuss how these terms are used in each of the contexts below. Use a maximum of three sentences per context. (8 points) a. When describing a compound: When describing a compound, the term electrolyte refers to the c ...
EXAM IIR - Academics
... 20. In another, parallel universe, the charge/mass ratio of a fundamental particle was measured and found to be + 5.685 x 10-12 coulombs/kg. From this one can conclude that: (A) The mass of the particle must be very large and/or the charge must be very small. (B) The particle has a net negative char ...
... 20. In another, parallel universe, the charge/mass ratio of a fundamental particle was measured and found to be + 5.685 x 10-12 coulombs/kg. From this one can conclude that: (A) The mass of the particle must be very large and/or the charge must be very small. (B) The particle has a net negative char ...
Kool Colors
... may turn black or may show signs of rust. Steel is primarily made from iron, and, when iron oxidizes, we call it rust. If something loses electrons, something else must gain those electrons. In this case, the dye in the Kool-Aid solution is gaining electrons and is said to be reduced. When the dye r ...
... may turn black or may show signs of rust. Steel is primarily made from iron, and, when iron oxidizes, we call it rust. If something loses electrons, something else must gain those electrons. In this case, the dye in the Kool-Aid solution is gaining electrons and is said to be reduced. When the dye r ...
PPT - Gmu - George Mason University
... E – total internal energy; the sum of kinetic and potential energies in the system q – heat flow between system and surroundings (-q indicates that heat is lost to surroundings) w – work (-w indicates work is lost to surroundings) H – Enthalpy – extensive property dependent on quantity of substance ...
... E – total internal energy; the sum of kinetic and potential energies in the system q – heat flow between system and surroundings (-q indicates that heat is lost to surroundings) w – work (-w indicates work is lost to surroundings) H – Enthalpy – extensive property dependent on quantity of substance ...
IChO_Comp_Prob_Answ 1997
... Montréal, Canada in July 1997. There are some areas of emphasis which certainly go beyond the routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even ...
... Montréal, Canada in July 1997. There are some areas of emphasis which certainly go beyond the routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even ...
STOICHIOMETRY
... of 96.7 % was obtained. What is the actual yield of copper II oxide in grams? Ans: 56.7 g 2. The percent yield for the reaction : PCl3 + Cl2 PCl5 is 74.3 %. What mass of product in grams, can be obtained from the reaction of 50.0 g Cl2 and an excess of PCl3 ? Ans: 108 g 3. The % yield for the reac ...
... of 96.7 % was obtained. What is the actual yield of copper II oxide in grams? Ans: 56.7 g 2. The percent yield for the reaction : PCl3 + Cl2 PCl5 is 74.3 %. What mass of product in grams, can be obtained from the reaction of 50.0 g Cl2 and an excess of PCl3 ? Ans: 108 g 3. The % yield for the reac ...
CHAPTER 21 NONMETALLIC ELEMENTS AND THEIR COMPOUNDS
... The density of a gas depends on temperature, pressure, and the molar mass of the substance. When two gases are at the same pressure and temperature, the ratio of their densities should be the same as the ratio of their molar masses. The molar mass of ammonium chloride is 53.5 g/mol, and the ratio of ...
... The density of a gas depends on temperature, pressure, and the molar mass of the substance. When two gases are at the same pressure and temperature, the ratio of their densities should be the same as the ratio of their molar masses. The molar mass of ammonium chloride is 53.5 g/mol, and the ratio of ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.