here
... hypothesis that maggots are created from rotting meat. Once a hypothesis has been formulated, scientists test it with more rigorous experiments. For example, after forming the hypothesis that maggots are created from rotting meat, early scientists did experiments to make sure that the maggots were n ...
... hypothesis that maggots are created from rotting meat. Once a hypothesis has been formulated, scientists test it with more rigorous experiments. For example, after forming the hypothesis that maggots are created from rotting meat, early scientists did experiments to make sure that the maggots were n ...
AP Chemistry Notes and Worksheets 2014
... o Ex. OH for water with O having a mass of 8 and H having a mass of 1 Avogadro's Hypothesis- Gay Lussac and Avogadro studied the volumes of combining gases. This allowed them to determine correct formulas. At the same temperature and pressure, equal volumes of different gases contain the same numb ...
... o Ex. OH for water with O having a mass of 8 and H having a mass of 1 Avogadro's Hypothesis- Gay Lussac and Avogadro studied the volumes of combining gases. This allowed them to determine correct formulas. At the same temperature and pressure, equal volumes of different gases contain the same numb ...
52 - University of Strathclyde
... (NHCs), this study reports the isolation of the first intermediates of alkali-metal-mediated zincation (AMMZn) of a free NHC and a Zn–NHC complex using sodium zincate [(TMEDA)NaZn(TMP)(tBu)2] (1) as a metallating reagent. The structural authentication of (THF)3Na[:C{[N(2,6-iPr2C6H3)]2CHCZn(tBu2)}] (2 ...
... (NHCs), this study reports the isolation of the first intermediates of alkali-metal-mediated zincation (AMMZn) of a free NHC and a Zn–NHC complex using sodium zincate [(TMEDA)NaZn(TMP)(tBu)2] (1) as a metallating reagent. The structural authentication of (THF)3Na[:C{[N(2,6-iPr2C6H3)]2CHCZn(tBu2)}] (2 ...
Chemical Equations Chemical Reaction: Interaction between
... Chemical Reaction: Interaction between substances that results in one or more new substances being produced Example: hydrogen + oxygen Æ water Reactants of a Reaction: Starting materials that undergo chemical change; written on the left side of the equation representing the reaction Products of a Re ...
... Chemical Reaction: Interaction between substances that results in one or more new substances being produced Example: hydrogen + oxygen Æ water Reactants of a Reaction: Starting materials that undergo chemical change; written on the left side of the equation representing the reaction Products of a Re ...
Chapter 5 ppt
... Chemical Reaction: Interaction between substances that results in one or more new substances being produced Example: hydrogen + oxygen water ...
... Chemical Reaction: Interaction between substances that results in one or more new substances being produced Example: hydrogen + oxygen water ...
Topic 1: Quantitative chemistry (12
... Include [Fe(H2O)6]3+, [Fe(CN)6]3–, [CuCl4]2– and [Ag(NH3)2]+. Only monodentate ligands are required. ...
... Include [Fe(H2O)6]3+, [Fe(CN)6]3–, [CuCl4]2– and [Ag(NH3)2]+. Only monodentate ligands are required. ...
Topic 1: Quantitative chemistry (12
... Include [Fe(H2O)6]3+, [Fe(CN)6]3–, [CuCl4]2– and [Ag(NH3)2]+. Only monodentate ligands are required. ...
... Include [Fe(H2O)6]3+, [Fe(CN)6]3–, [CuCl4]2– and [Ag(NH3)2]+. Only monodentate ligands are required. ...
Spillover in Heterogeneous Catalysis - ACS Publications
... Ill.Spillover Processes in Catalysis Several types of processes have been described that involve the participation of spillover in catalysis. The first systems where spillover was detected were supported metals with hydrogen spillover from the metal to the support. It was found that species could sp ...
... Ill.Spillover Processes in Catalysis Several types of processes have been described that involve the participation of spillover in catalysis. The first systems where spillover was detected were supported metals with hydrogen spillover from the metal to the support. It was found that species could sp ...
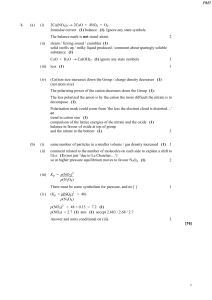
1. (a) (i) 2Ca(NO3)2 → 2CaO + 4NO2 + O2 formulae correct (1
... • Recognizing the existence of hydrogen bonds ( between molecules) (1) • That each molecule can form more than one hydrogen bond because of the two OH (and two S=O groups) / or a description of hydrogen bonds in this case / or a diagram showing the hydrogen bonds (1) ...
... • Recognizing the existence of hydrogen bonds ( between molecules) (1) • That each molecule can form more than one hydrogen bond because of the two OH (and two S=O groups) / or a description of hydrogen bonds in this case / or a diagram showing the hydrogen bonds (1) ...
advanced placement chemistry workbook and note set
... electrons and form ions, which are electrically-charged chemical species. The identity of the element does not change – only the electrical charge of the species changes. Recall from first-year chemistry that atoms lose or gain electrons based upon their metal or nonmetal character, and that the num ...
... electrons and form ions, which are electrically-charged chemical species. The identity of the element does not change – only the electrical charge of the species changes. Recall from first-year chemistry that atoms lose or gain electrons based upon their metal or nonmetal character, and that the num ...
1. True
... 1. −222.0 kJ · mol−1 2. −222, 000 kJ · mol−1 3. −22.20 kJ · mol−1 4. −22, 200 kJ · mol−1 5. −2, 220 kJ · mol−1 ΔT = Tf − Ti = 77.96 ◦C − 24.90 ◦C = 53.06 ◦C = 53.06 K m = 1 L ·(1000 mL/L)·(1.00 g/mL) = 1000 g n = 4.409 g propane ·(1mol/44.09 g) = 0.1 mol propane −ΔHrxn = ΔHcal = mcΔT = 1000 g · 4.18 ...
... 1. −222.0 kJ · mol−1 2. −222, 000 kJ · mol−1 3. −22.20 kJ · mol−1 4. −22, 200 kJ · mol−1 5. −2, 220 kJ · mol−1 ΔT = Tf − Ti = 77.96 ◦C − 24.90 ◦C = 53.06 ◦C = 53.06 K m = 1 L ·(1000 mL/L)·(1.00 g/mL) = 1000 g n = 4.409 g propane ·(1mol/44.09 g) = 0.1 mol propane −ΔHrxn = ΔHcal = mcΔT = 1000 g · 4.18 ...
Answers - logo Pre-U Chemistry Textbook
... Two electrons would have to go into an antibonding molecular orbital. This is energetically unfavourable because the increase in energy of the antibonding orbital is greater than the decrease in energy of the bonding orbital. Helium molecules do not form because they would be at higher energy than t ...
... Two electrons would have to go into an antibonding molecular orbital. This is energetically unfavourable because the increase in energy of the antibonding orbital is greater than the decrease in energy of the bonding orbital. Helium molecules do not form because they would be at higher energy than t ...
Stoichiometry worksheet KEY
... e) Use the answers from questions b, c, and d above to show that this equation obeys the law of conservation of mass. Mass of reactants = mass of products (52.0 g C2H2 + 160 g O2) = (176 g CO2 + 36.0 g H2O) 212 g reactants = 212 g products ...
... e) Use the answers from questions b, c, and d above to show that this equation obeys the law of conservation of mass. Mass of reactants = mass of products (52.0 g C2H2 + 160 g O2) = (176 g CO2 + 36.0 g H2O) 212 g reactants = 212 g products ...
The Uptake of Methyl Vinyl Ketone
... the formation of secondary organic aerosols,1-5 in particular, for precursors for which gas-phase oxidation does not otherwise produce aerosols, such as isoprene and its oxidation products.4 Various explanations for these observations have been proposed, most emphasizing that the increase of aerosol ...
... the formation of secondary organic aerosols,1-5 in particular, for precursors for which gas-phase oxidation does not otherwise produce aerosols, such as isoprene and its oxidation products.4 Various explanations for these observations have been proposed, most emphasizing that the increase of aerosol ...
x - SharpSchool
... between H2(g) and I2(g) (simple molecules and takes place in gas phase no solvent necessary!) H2(g) + I2(g) ⇌ 2 HI(g) ...
... between H2(g) and I2(g) (simple molecules and takes place in gas phase no solvent necessary!) H2(g) + I2(g) ⇌ 2 HI(g) ...
1. dia
... • Sulfur trioxide at 482 oC transforms to sulfuric acid. Under the dew point sulfuric acid condensates on the structure materials (heat exchanger, stack wall ). The dew point of sulfuric acid depends on the SO3 and water content of the stack ...
... • Sulfur trioxide at 482 oC transforms to sulfuric acid. Under the dew point sulfuric acid condensates on the structure materials (heat exchanger, stack wall ). The dew point of sulfuric acid depends on the SO3 and water content of the stack ...
TOPIC 11 Further equilibrium 11.1 Chemical equilibrium
... If the pressure is increased then the volume of the reaction mixture will decrease. This will result in an instantaneous equal increase in the concentration of each component. However, the increase in the magnitude of [CH3OH(g)][H2(g)]2 will be greater than the increase in the magnitude of [CH3OH(g) ...
... If the pressure is increased then the volume of the reaction mixture will decrease. This will result in an instantaneous equal increase in the concentration of each component. However, the increase in the magnitude of [CH3OH(g)][H2(g)]2 will be greater than the increase in the magnitude of [CH3OH(g) ...
431 KB / 47 pages
... (a) We have seen (Investigate This 10.2) that electrolysis of a dilute aqueous solution of an ionic compound (magnesium sulfate) produces a gas at both electrodes and a basic solution at the cathode and acidic solution at the anode, just as the problem statement says is observed here for a dilute aq ...
... (a) We have seen (Investigate This 10.2) that electrolysis of a dilute aqueous solution of an ionic compound (magnesium sulfate) produces a gas at both electrodes and a basic solution at the cathode and acidic solution at the anode, just as the problem statement says is observed here for a dilute aq ...
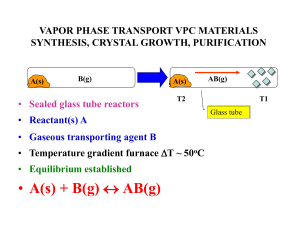
vapor phase transport vpc materials synthesis, crystal growth
... The reactor consists of three inner quartz tubes, which supply the reactive gases, InCl3, GaCl3 (N2 carrier) and NH3, and an outer quartz tube, which supplies inert gas (N2) and houses the reaction in a horizontal tube furnace. Two independently controlled heating tapes were used to tune the vapour ...
... The reactor consists of three inner quartz tubes, which supply the reactive gases, InCl3, GaCl3 (N2 carrier) and NH3, and an outer quartz tube, which supplies inert gas (N2) and houses the reaction in a horizontal tube furnace. Two independently controlled heating tapes were used to tune the vapour ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.