Microbial Metabolism
... • Needed for aerobic metabolism (cytochromes, iron-sulfur proteins) • Insoluble under aerobic conditions – Fe(OH)3, FeOOH – Solubilized by siderophores ...
... • Needed for aerobic metabolism (cytochromes, iron-sulfur proteins) • Insoluble under aerobic conditions – Fe(OH)3, FeOOH – Solubilized by siderophores ...
Lecture 9. Redox chemistry
... - A gold ring is accidentally dropped into a solution of hydrochloric acid No reaction occurs, gold is below hydrogen on the activity series. ...
... - A gold ring is accidentally dropped into a solution of hydrochloric acid No reaction occurs, gold is below hydrogen on the activity series. ...
S2-2-07 - Classifying Chemical Reactions
... from notes. Think of other analogies for this type of reaction and fill in notes sheet. Teacher’s analogy: Blackeye + Pinkpanther Blackpanther + Pinkeye. (10 minutes) Have students follow the directions found with material at the center of their table and perform the double displacement reaction B ...
... from notes. Think of other analogies for this type of reaction and fill in notes sheet. Teacher’s analogy: Blackeye + Pinkpanther Blackpanther + Pinkeye. (10 minutes) Have students follow the directions found with material at the center of their table and perform the double displacement reaction B ...
chemical reaction
... (aq) – aqueous (dissolved in water, exists as ions) ↓ - a precipitate has formed ...
... (aq) – aqueous (dissolved in water, exists as ions) ↓ - a precipitate has formed ...
syllabus for entrance examination - NTU.edu
... Simple calculations on half-life may be set. Questions will not be set requiring candidates to determine the order of a reaction using methods that involve drawing tangents to curves. Candidates will not be asked to determine the values of rate constants but should be able to construct a rate equati ...
... Simple calculations on half-life may be set. Questions will not be set requiring candidates to determine the order of a reaction using methods that involve drawing tangents to curves. Candidates will not be asked to determine the values of rate constants but should be able to construct a rate equati ...
Viju B - IS MU
... Department of Chemistry, Faculty of Science, Masaryk University, Kamenice 5/A8, 625 00, Brno, Czech Republic ...
... Department of Chemistry, Faculty of Science, Masaryk University, Kamenice 5/A8, 625 00, Brno, Czech Republic ...
Chemical Reactions and Equations
... when they are left exposed to air for a long time. This is known as rancidity. Rancidity is caused due to oxidation of fat and oil present in food materials. Rancidity can be prevented by using various methods such as by adding antioxidants to the food ...
... when they are left exposed to air for a long time. This is known as rancidity. Rancidity is caused due to oxidation of fat and oil present in food materials. Rancidity can be prevented by using various methods such as by adding antioxidants to the food ...
Chemical Reactions and Equations
... when they are left exposed to air for a long time. This is known as rancidity. Rancidity is caused due to oxidation of fat and oil present in food materials. Rancidity can be prevented by using various methods such as by adding antioxidants to the food materials, storing food in air tight container ...
... when they are left exposed to air for a long time. This is known as rancidity. Rancidity is caused due to oxidation of fat and oil present in food materials. Rancidity can be prevented by using various methods such as by adding antioxidants to the food materials, storing food in air tight container ...
Chemistry 100
... completion? Each involves the reaction symbolized by the equation: N2(g) + 3H2(g) → 2NH3(g) A) B) C) D) ...
... completion? Each involves the reaction symbolized by the equation: N2(g) + 3H2(g) → 2NH3(g) A) B) C) D) ...
Chemical Reactions
... only C, H, (and maybe O) is reacted with oxygen – usually called “burning” If the combustion is complete, the products will be CO2 and H2O. If the combustion is incomplete, the products will be CO (or possibly just C) and H2O. ...
... only C, H, (and maybe O) is reacted with oxygen – usually called “burning” If the combustion is complete, the products will be CO2 and H2O. If the combustion is incomplete, the products will be CO (or possibly just C) and H2O. ...
Final Review: L17-25
... Attraction strength between molecules determine some of the liquid properties, such as vapor pressure, viscosity, and surface tension. ...
... Attraction strength between molecules determine some of the liquid properties, such as vapor pressure, viscosity, and surface tension. ...
MS PowerPoint - Catalysis Eprints database
... A catalyst is a material that favorably influences a reaction but emerges from the reaction unchanged ...
... A catalyst is a material that favorably influences a reaction but emerges from the reaction unchanged ...
CHEMISTry is life - World of Teaching
... they are scared before they even begin. -My goal is to shape a positive image in their minds about chemistry so that they can be more prepared mentally for high school. -I will do this by showing them how applicable chemistry is to every day life. It is the study of everything, and it is the central ...
... they are scared before they even begin. -My goal is to shape a positive image in their minds about chemistry so that they can be more prepared mentally for high school. -I will do this by showing them how applicable chemistry is to every day life. It is the study of everything, and it is the central ...
Chemistry Standards Review
... Atoms combine to form molecules by sharing electrons to form covalent or metallic bonds or by exchanging electrons to form ionic bonds. The chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are ionic. Large molecules (polymers), such ...
... Atoms combine to form molecules by sharing electrons to form covalent or metallic bonds or by exchanging electrons to form ionic bonds. The chemical bonds between atoms in molecules such as H2, CH4, NH3, H2CCH2, N2, Cl2, and many large biological molecules are ionic. Large molecules (polymers), such ...
Chemical Equations and Reactions
... The negative ion of one compound replaces the negative ion of the other compound to form 2 new compounds. Usually forms a precipitate, water or a gas. ...
... The negative ion of one compound replaces the negative ion of the other compound to form 2 new compounds. Usually forms a precipitate, water or a gas. ...
AP Chemistry
... 1. Acid-base reactions; concepts of Arrhenius, Brønsted-Lowry, Lewis; coordination complexes; amphoterism 2. Precipitation reactions 3. Oxidation-reduction reactions a. Oxidation number, b. The role of the electron in oxidation-reduction, c. Electrochemistry: electrolytic and galvanic cells; Faraday ...
... 1. Acid-base reactions; concepts of Arrhenius, Brønsted-Lowry, Lewis; coordination complexes; amphoterism 2. Precipitation reactions 3. Oxidation-reduction reactions a. Oxidation number, b. The role of the electron in oxidation-reduction, c. Electrochemistry: electrolytic and galvanic cells; Faraday ...
CHEMISTRY
... Also called displacement Many are in aqueous solutions Less E required The more active element replaces the less active one Most active metals (group 1) react w/water and produce metal hydroxides ...
... Also called displacement Many are in aqueous solutions Less E required The more active element replaces the less active one Most active metals (group 1) react w/water and produce metal hydroxides ...
Reaction rate and activation energy of the acidolysis
... 1.0 molar sodium hydroxide solution into a 1000 ml volumetric flask and filling up to the calibration mark with water. Fill the burette with 0.2 molar NaOH solution. Pipette 100 ml of 0.1 molar hydrochloric acid solution into an Erlenmeyer flask, seal it with a stopper, and temperature equilibrate i ...
... 1.0 molar sodium hydroxide solution into a 1000 ml volumetric flask and filling up to the calibration mark with water. Fill the burette with 0.2 molar NaOH solution. Pipette 100 ml of 0.1 molar hydrochloric acid solution into an Erlenmeyer flask, seal it with a stopper, and temperature equilibrate i ...
PDF(343KB)
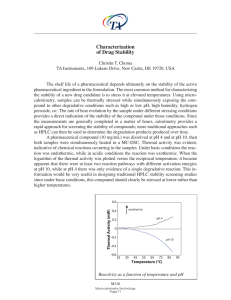
... the stability of a new drug candidate is to stress it at elevated temperatures. Using microcalorimetry, samples can be thermally stressed while simultaneously exposing the compound to other degradative conditions such as high or low pH, high humidity, hydrogen peroxide, etc. The rate of heat evoluti ...
... the stability of a new drug candidate is to stress it at elevated temperatures. Using microcalorimetry, samples can be thermally stressed while simultaneously exposing the compound to other degradative conditions such as high or low pH, high humidity, hydrogen peroxide, etc. The rate of heat evoluti ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.