NYOS Charter School
... 14. Which of the following is true of equilibria reactions in chemistry? a. reactions with a large, positive Keq proceed very quickly b. all particle movement stops when equilibrium is reached c. particles are continuously moving back and forth between reactants and products d. the state of equilibr ...
... 14. Which of the following is true of equilibria reactions in chemistry? a. reactions with a large, positive Keq proceed very quickly b. all particle movement stops when equilibrium is reached c. particles are continuously moving back and forth between reactants and products d. the state of equilibr ...
MCQ plus answers
... An enzyme is a protein that is a highly efficient catalyst for one or more chemical reactions in a living system. ...
... An enzyme is a protein that is a highly efficient catalyst for one or more chemical reactions in a living system. ...
1 Introduction
... and/or transporting these highly toxic reagents is problematic. To understand this better, let us look at two examples: hydrogen-powered fuel cells and oxidation of propene to propene oxide. Hydrogen-powered fuel cells are a hot topic. In principle, such fuel cells can provide us with energy, whil ...
... and/or transporting these highly toxic reagents is problematic. To understand this better, let us look at two examples: hydrogen-powered fuel cells and oxidation of propene to propene oxide. Hydrogen-powered fuel cells are a hot topic. In principle, such fuel cells can provide us with energy, whil ...
PowerPoint for Cornell Notes
... The substance gaining electrons is said to be reduced and the substance losing the electrons is said to be oxidized. Thus an oxidation reaction is called a Redox reaction. In the Redox Reaction diagram, sodium (Na) is being oxidized and chlorine (Cl) is being reduced. The term "oxidation", with its ...
... The substance gaining electrons is said to be reduced and the substance losing the electrons is said to be oxidized. Thus an oxidation reaction is called a Redox reaction. In the Redox Reaction diagram, sodium (Na) is being oxidized and chlorine (Cl) is being reduced. The term "oxidation", with its ...
Solution
... = 1.83 x 1083, this is a very large K indicating that the products are strongly favored. This is consistent with the negative free energy of part (c). e) The pressure of oxygen is 5 atm and the pressure of hydrogen is 10 atm at 25°C. In which direction will the reaction shift in order to regain equi ...
... = 1.83 x 1083, this is a very large K indicating that the products are strongly favored. This is consistent with the negative free energy of part (c). e) The pressure of oxygen is 5 atm and the pressure of hydrogen is 10 atm at 25°C. In which direction will the reaction shift in order to regain equi ...
syllabus for screening test (mcq type)
... Concentration dependence of rate of reaction, differential and integral rate equations for zeroth, first, second order reactions, rate equations involving reverse, parallel, consecutive and chain reactions, effect of temperature and pressure on rate constant, collision and transition state theories ...
... Concentration dependence of rate of reaction, differential and integral rate equations for zeroth, first, second order reactions, rate equations involving reverse, parallel, consecutive and chain reactions, effect of temperature and pressure on rate constant, collision and transition state theories ...
Test 2
... 13. A sample of limestone (containing calcium carbonate, CaCO3) weighing 413mg is treated with oxalic acid (H2C2O4) to give 472mg calcium oxalate (CaC2O4). CaCO3(s) + H2C2O4(aq) CaC2O4(s) + CO2(g) + H2O(l). What is the percentage of calcium carbonate in the limestone? Compound CaCO3 H2C2O4 CaC2O4 ...
... 13. A sample of limestone (containing calcium carbonate, CaCO3) weighing 413mg is treated with oxalic acid (H2C2O4) to give 472mg calcium oxalate (CaC2O4). CaCO3(s) + H2C2O4(aq) CaC2O4(s) + CO2(g) + H2O(l). What is the percentage of calcium carbonate in the limestone? Compound CaCO3 H2C2O4 CaC2O4 ...
Chemistry Quiz #2 Study Guide (Answers)
... • Evaporation – The process by which a substance changes from its liquid state to its gas state. (endothermic) • Condensation – The process by which a gas becomes a liquid. (exothermic) • Solidification – The process by which a liquid becomes a solid. (exothermic) • Deposition – The process by which ...
... • Evaporation – The process by which a substance changes from its liquid state to its gas state. (endothermic) • Condensation – The process by which a gas becomes a liquid. (exothermic) • Solidification – The process by which a liquid becomes a solid. (exothermic) • Deposition – The process by which ...
Chapter 8powerp point for chemical reactions
... Solid calcium metal reacts with water to form aqueous calcium hydroxide and hydrogen gas. ...
... Solid calcium metal reacts with water to form aqueous calcium hydroxide and hydrogen gas. ...
Unit 5 Chemical Properties and Changes Video Notes A ______ is a
... ________________________ A change that alters the identity of a substance resulting in a new substance or substances with different properties ________________________ Those characteristics that can be observed when a chemical reaction changes the identity of the substance, such as potential to rus ...
... ________________________ A change that alters the identity of a substance resulting in a new substance or substances with different properties ________________________ Those characteristics that can be observed when a chemical reaction changes the identity of the substance, such as potential to rus ...
Direct production of hydrogen peroxide from CO, O2, and H2O over
... for highly selectively converting organic compounds into value-added products, as well as for industrial or municipal wastewater treatment and water disinfection.1 Currently, the commercial production of H2O2 is mainly based on a multistep process involving cyclic hydrogenation and oxidation of an a ...
... for highly selectively converting organic compounds into value-added products, as well as for industrial or municipal wastewater treatment and water disinfection.1 Currently, the commercial production of H2O2 is mainly based on a multistep process involving cyclic hydrogenation and oxidation of an a ...
Single-Replacement Reactions
... This is the only type of chemical reaction in which there is a single product formed. This single product is always more complex than the reactants. ...
... This is the only type of chemical reaction in which there is a single product formed. This single product is always more complex than the reactants. ...
2014
... Rate = k[M]1[N]2 5) The rate of a certain chemical reaction between substances M and N obeys the rate law above. The reaction is studied with [M] and [N] each 1 x 10-3M. If a new experiment is conducted with [M] and [N] each 2 x 10-3M, the reaction rate will increase by a factor of (A)2 (B)4 (C)6 ( ...
... Rate = k[M]1[N]2 5) The rate of a certain chemical reaction between substances M and N obeys the rate law above. The reaction is studied with [M] and [N] each 1 x 10-3M. If a new experiment is conducted with [M] and [N] each 2 x 10-3M, the reaction rate will increase by a factor of (A)2 (B)4 (C)6 ( ...
acids and bases - No Brain Too Small
... You get given a table of ions but need to learn their names ...
... You get given a table of ions but need to learn their names ...
Chemistry Standards Review
... 18. Which of the following statements about temperature and molecular motion is NOT true? (A) Temperature is determined by the average kinetic energy of particles (B) Molar heat capacity is related to the specific heat of a substance (C) Entropy is related to concentration (D) Temperature is determ ...
... 18. Which of the following statements about temperature and molecular motion is NOT true? (A) Temperature is determined by the average kinetic energy of particles (B) Molar heat capacity is related to the specific heat of a substance (C) Entropy is related to concentration (D) Temperature is determ ...
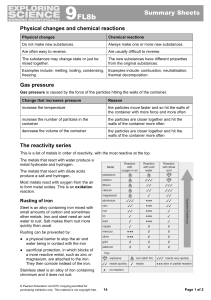
9F Reactivity - Parrs Wood High School
... metal hydroxide and hydrogen. The metals that react with dilute acids produce a salt and hydrogen. Most metals react with oxygen from the air to form metal oxides. This is an oxidation reaction. ...
... metal hydroxide and hydrogen. The metals that react with dilute acids produce a salt and hydrogen. Most metals react with oxygen from the air to form metal oxides. This is an oxidation reaction. ...
Catalysis

Catalysis is the increase in the rate of a chemical reaction due to the participation of an additional substance called a catalyst. With a catalyst, reactions occur faster and require less activation energy. Because catalysts are not consumed in the catalyzed reaction, they can continue to catalyze the reaction of further quantities of reactant. Often only tiny amounts are required.