Name______________________ Period________
... What is the new pressure that the gas will exert, if the temperature is raised to 300.0 °C? (Assume volume is constant) (SHOW WORK) ...
... What is the new pressure that the gas will exert, if the temperature is raised to 300.0 °C? (Assume volume is constant) (SHOW WORK) ...
Strong and weak acids and bases
... groups, COOH): – methanoic acid (CHOOH) – ethanoic (acetic) acid • CH3COOH(l) + H2O(l) ⇌ CH3COO-(aq) + H3O+ – carbonic acid: (CO2 in water) • CO2(aq) + H2O(l) ⇌ H2CO3(aq) ...
... groups, COOH): – methanoic acid (CHOOH) – ethanoic (acetic) acid • CH3COOH(l) + H2O(l) ⇌ CH3COO-(aq) + H3O+ – carbonic acid: (CO2 in water) • CO2(aq) + H2O(l) ⇌ H2CO3(aq) ...
Honors Chemistry Final Essay Questions 2007
... label the states of the reactants and products on both the balanced equation and the net ionic equation, and list the spectator ions and driving force for each of the reactions. a. silver nitrate solution + magnesium bromide solution b. barium chloride solution + potassium sulfate solution c. sulfur ...
... label the states of the reactants and products on both the balanced equation and the net ionic equation, and list the spectator ions and driving force for each of the reactions. a. silver nitrate solution + magnesium bromide solution b. barium chloride solution + potassium sulfate solution c. sulfur ...
Density of solutions answers The concentration of solutions is often
... If the temperature of 85.2g of water increases from 25.2 temperature to 37.6 temperature, how much heat was absorbed by the water? What is the enthalpy change for a reaction, and how does this differ from the experimental heat flow measured for an experiment involving the reaction. Use your textbook ...
... If the temperature of 85.2g of water increases from 25.2 temperature to 37.6 temperature, how much heat was absorbed by the water? What is the enthalpy change for a reaction, and how does this differ from the experimental heat flow measured for an experiment involving the reaction. Use your textbook ...
The origin and status of the Arrhenius equation
... an appreciable dependence on temperature, but for the next few decades, kinetic studies were directed more toward quantifying the effect of reactant concentrations (2) rather than that of temperature. Several sets of data on the latter effect were ohtained in the eighteen-eighties, and in 1889, Svau ...
... an appreciable dependence on temperature, but for the next few decades, kinetic studies were directed more toward quantifying the effect of reactant concentrations (2) rather than that of temperature. Several sets of data on the latter effect were ohtained in the eighteen-eighties, and in 1889, Svau ...
GR 7.1 Understanding Solutions Guided Reading and Study Use
... tea must have been a saturated solution. unsaturated solution: On the other hand, my uncle makes a weak sweetened tea that surely must be an unsaturaed solution. supersaturated solution: If you heated my aunt’s sweet tea, I supose you might get a bit more sugar to dissolve in it, making it a supersa ...
... tea must have been a saturated solution. unsaturated solution: On the other hand, my uncle makes a weak sweetened tea that surely must be an unsaturaed solution. supersaturated solution: If you heated my aunt’s sweet tea, I supose you might get a bit more sugar to dissolve in it, making it a supersa ...
2017 Chemistry Exam Review Compounds and Reactions 1. Know
... 22. What is meant by system vs. surroundings? What is true about energy for a system plus its surroundings? 23. How does heat relate to thermal energy? 24. In which direction (in terms of temperature) does heat move spontaneously? In terms of the way molecules collide with each other, why is this so ...
... 22. What is meant by system vs. surroundings? What is true about energy for a system plus its surroundings? 23. How does heat relate to thermal energy? 24. In which direction (in terms of temperature) does heat move spontaneously? In terms of the way molecules collide with each other, why is this so ...
Inorganic Chemistry
... cycle and its efficiency, Carnot theorem. Thermodynamic scale of temperature. Concept of entropy: entropy as a state function, entropy as a function of V and T, entropy as a function of P and T, entropy change in physical and chemical processes, entropy change in reversible and irreversible processe ...
... cycle and its efficiency, Carnot theorem. Thermodynamic scale of temperature. Concept of entropy: entropy as a state function, entropy as a function of V and T, entropy as a function of P and T, entropy change in physical and chemical processes, entropy change in reversible and irreversible processe ...
Solubility
... – Waters of Earth contains dissolved salts as water passes over and through the ground – Ppt of CaCO3 from groundwater is responsible for cave formation. Let’s look at the factors that affect solubility! ...
... – Waters of Earth contains dissolved salts as water passes over and through the ground – Ppt of CaCO3 from groundwater is responsible for cave formation. Let’s look at the factors that affect solubility! ...
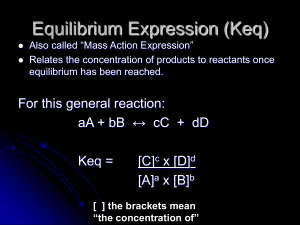
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.