Chemical Equations & Reactions
... Determine the heat of reaction, ΔH, (enthalpy change) for this reaction. Determine the activation energy, Ea for this reaction. How much energy is released or absorbed during the reaction? How much energy is required for this reaction to occur? ...
... Determine the heat of reaction, ΔH, (enthalpy change) for this reaction. Determine the activation energy, Ea for this reaction. How much energy is released or absorbed during the reaction? How much energy is required for this reaction to occur? ...
CHEMISTRY-1 CHAPTER 8 CHEMICAL REACTIONS
... • Predict the products. Write and balance the following synthesis reaction equations. • Sodium metal reacts with chlorine gas 2 Na(s) + Cl2(g) 2NaCl(s) • Solid Magnesium reacts with fluorine gas Mg(s) + F2(g) MgF2(s) • Aluminum metal reacts with fluorine gas 2Al(s) + 3F2(g) 2 AlF3(s) ...
... • Predict the products. Write and balance the following synthesis reaction equations. • Sodium metal reacts with chlorine gas 2 Na(s) + Cl2(g) 2NaCl(s) • Solid Magnesium reacts with fluorine gas Mg(s) + F2(g) MgF2(s) • Aluminum metal reacts with fluorine gas 2Al(s) + 3F2(g) 2 AlF3(s) ...
advanced placement chemistry alamo heights high school scope
... This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. For most students, the course enables them to undertake, as a ...
... This AP Chemistry course is designed to be the equivalent of the general chemistry course usually taken during the first year of college. For most students, the course enables them to undertake, as a ...
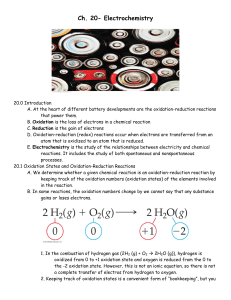
Ch. 20- Electrochemistry
... the -2 oxidation state. However, this is not an ionic equation, so there is not a complete transfer of electros from hydrogen to oxygen. 2. Keeping track of oxidation states is a convenient form of “bookkeeping”, but you ...
... the -2 oxidation state. However, this is not an ionic equation, so there is not a complete transfer of electros from hydrogen to oxygen. 2. Keeping track of oxidation states is a convenient form of “bookkeeping”, but you ...
spontaneous processes
... requires more than just an “undo” -- we can restore the original system, but the surroundings will have changed ...
... requires more than just an “undo” -- we can restore the original system, but the surroundings will have changed ...
Reaction Kinetics. The Bromination of Acetone
... Determination of the B constant: The constant B of equation [10] is determined by measuring the absorbance of at least three solutions of known bromine concentration. At room temperature, prepare one solution by pipetting 10.0 ml of stock 0.02 M Br2 into a clean 125-ml Erlenmeyer flask. Add 10.0 ml ...
... Determination of the B constant: The constant B of equation [10] is determined by measuring the absorbance of at least three solutions of known bromine concentration. At room temperature, prepare one solution by pipetting 10.0 ml of stock 0.02 M Br2 into a clean 125-ml Erlenmeyer flask. Add 10.0 ml ...
ksp - lozon.ca
... induced to come to equilibrium by the addition of a "seed" which may be a tiny crystal of the solute, or a tiny solid particle, which initiates precipitation. This equilibrium constant is dimensionless as activity is a dimensionless quantity. However, use of activities is very inconvenient, so the e ...
... induced to come to equilibrium by the addition of a "seed" which may be a tiny crystal of the solute, or a tiny solid particle, which initiates precipitation. This equilibrium constant is dimensionless as activity is a dimensionless quantity. However, use of activities is very inconvenient, so the e ...
Carbon Dioxide, Carbonic Acid, and Carbonate Equilibria
... Carbonate Equilibria and pH The following graph shows the variation in [H2CO3], [HCO3-], and [CO32-] with changes in pH. ...
... Carbonate Equilibria and pH The following graph shows the variation in [H2CO3], [HCO3-], and [CO32-] with changes in pH. ...
NCEA Level 3 Chemistry (91392) 2015
... The pH of a solution is calculated from its [H3O+]. NaOH is an ionic solid that is a strong base and dissociates completely to produce a high OH– concentration (low [H3O+]). Since [OH–] is high / [H3O+] is low, the pH is high. NaOH → Na+ + OH– CH3NH2 is a weak base that partially reacts / dissociate ...
... The pH of a solution is calculated from its [H3O+]. NaOH is an ionic solid that is a strong base and dissociates completely to produce a high OH– concentration (low [H3O+]). Since [OH–] is high / [H3O+] is low, the pH is high. NaOH → Na+ + OH– CH3NH2 is a weak base that partially reacts / dissociate ...
(III) From Aqueous Solutions with Sodium Dodecyl Sulfate
... materials, x-ray films. So their prices are ten times higher in comparison with the total product. For REE extraction from solutions leaching of the ore concentrates using technology based on physic-chemical methods: extraction by organic reagents, ion exchange [4, 5]. It deals with the application ...
... materials, x-ray films. So their prices are ten times higher in comparison with the total product. For REE extraction from solutions leaching of the ore concentrates using technology based on physic-chemical methods: extraction by organic reagents, ion exchange [4, 5]. It deals with the application ...
Study Guide Chapter 16: The Process of Chemical Reactions
... If the bonds in the products are stronger and lower potential energy than in the reactants, energy will be released from the system. If the energy released is due to the conversion of potential energy to kinetic energy, the temperature of the products will be higher than the original reactants. The ...
... If the bonds in the products are stronger and lower potential energy than in the reactants, energy will be released from the system. If the energy released is due to the conversion of potential energy to kinetic energy, the temperature of the products will be higher than the original reactants. The ...
Lecture Notes through 8-29-06
... Organic chemistry received a boost when it was realized that these compounds could be treated in ways similar to inorganic compounds and could be manufactured by means other than 'vital force' Inorganic only simple carbon compounds, with no carbon to carbon connections (its oxides, acids, salts, ...
... Organic chemistry received a boost when it was realized that these compounds could be treated in ways similar to inorganic compounds and could be manufactured by means other than 'vital force' Inorganic only simple carbon compounds, with no carbon to carbon connections (its oxides, acids, salts, ...
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.