ICE Tables - Chemwiki
... Know the direction of the reaction. This knowledge will affect the "change" row of the ICE table (for our example, we knew the reaction would proceed forward, as there was no initial products). Direction of reaction can be calculated using Q, the reaction quotient, which is then compared to a known ...
... Know the direction of the reaction. This knowledge will affect the "change" row of the ICE table (for our example, we knew the reaction would proceed forward, as there was no initial products). Direction of reaction can be calculated using Q, the reaction quotient, which is then compared to a known ...
Project Details PPT
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
... oP R E V E N T W A S T E B Y U S I N G A SOLVENTLESS PROCESS. oM I N I M I Z E A M O U N T S O F S O L V E N T S A N D REAGENTS USED. ...
ABCT2772
... values in simple processes b. use the Thermodynamics principles and functions to analysis simple chemical systems and determine the effect of external conditions on their equilibrium positions. c. demonstrate a better understanding on the fundamental principles of reaction rate theories as well as t ...
... values in simple processes b. use the Thermodynamics principles and functions to analysis simple chemical systems and determine the effect of external conditions on their equilibrium positions. c. demonstrate a better understanding on the fundamental principles of reaction rate theories as well as t ...
Lecture 18. Chemical Equilibrium (Ch. 5)
... During this reaction, some bonds should be broken and other bonds (with a more negative potential energy) should be formed. This process is characterized by a potential barrier – thus, the Boltzmann factor! For the “direct” reaction, the barrier is r s Δ H , for the reverse one - Δ H . The reactions ...
... During this reaction, some bonds should be broken and other bonds (with a more negative potential energy) should be formed. This process is characterized by a potential barrier – thus, the Boltzmann factor! For the “direct” reaction, the barrier is r s Δ H , for the reverse one - Δ H . The reactions ...
equilibrium
... IS NOT equal to concentration of product The process is affected by temperature and pressure ...
... IS NOT equal to concentration of product The process is affected by temperature and pressure ...
Objectives - Dixie State University
... 2. Explain what is happening to the rates of the reactions and the concentrations of the reactants in both reactions when the reaction is at equilibrium. The Equilibrium Constant 3. Give the equation for the equilibrium constant, and explain what a large or small K eq means. Independence of rate and ...
... 2. Explain what is happening to the rates of the reactions and the concentrations of the reactants in both reactions when the reaction is at equilibrium. The Equilibrium Constant 3. Give the equation for the equilibrium constant, and explain what a large or small K eq means. Independence of rate and ...
CHAPTER 1-MATTER AND ITS PROPERTIES The
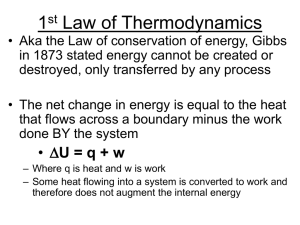
... 14. In thermodynamics __ work____ means the transfer of energy between the system and its surroundings due to an external makroscopic force. 15. Apart from transfer of heat, some chemical processes may do work.(for example: the ___expansion or ___compression___ of gases) 16. A system does not contai ...
... 14. In thermodynamics __ work____ means the transfer of energy between the system and its surroundings due to an external makroscopic force. 15. Apart from transfer of heat, some chemical processes may do work.(for example: the ___expansion or ___compression___ of gases) 16. A system does not contai ...
Standard 4 notes
... Conservation of Mass—Atoms are not created or destroyed in chemical reactions. They just change partners. If we could mass the reactants in a sealed, airtight container and make them react without opening the container, we should find that the total mass before the reaction is the same as the total ...
... Conservation of Mass—Atoms are not created or destroyed in chemical reactions. They just change partners. If we could mass the reactants in a sealed, airtight container and make them react without opening the container, we should find that the total mass before the reaction is the same as the total ...
The Buffer Equation
... Osmosis is defined as the passage of the solvent into a solution through a semipermeable membrane. This process tends to equalize the escaping tendency of the solvent on both sides of the membrane. Escaping tendency can be measured in terms of vapor pressure or the closely related colligative proper ...
... Osmosis is defined as the passage of the solvent into a solution through a semipermeable membrane. This process tends to equalize the escaping tendency of the solvent on both sides of the membrane. Escaping tendency can be measured in terms of vapor pressure or the closely related colligative proper ...
Document
... the vapour pressure of water constant at 3.17kPa, its heat capacityy 75.5 JK-1mol-1 and it behaves as a perfect gas. Assume that the temperature of air is constant. If you need any extra data to solve the problems, search for them in the Data section in the end of the Atkins book! ...
... the vapour pressure of water constant at 3.17kPa, its heat capacityy 75.5 JK-1mol-1 and it behaves as a perfect gas. Assume that the temperature of air is constant. If you need any extra data to solve the problems, search for them in the Data section in the end of the Atkins book! ...
Spring Benchmark Exam
... 30. A technician prepared a solution by heating 100 milliliters of distilled water while adding KCl crystals until no more KCl would dissolve. She then capped the clear solution and set it aside on the lab bench. After several hours she noticed the solution had become cloudy and some solid had settl ...
... 30. A technician prepared a solution by heating 100 milliliters of distilled water while adding KCl crystals until no more KCl would dissolve. She then capped the clear solution and set it aside on the lab bench. After several hours she noticed the solution had become cloudy and some solid had settl ...
Chapter 5 auxiliary functions
... T , we can write : Δ A + T Δ sirr = 0 For an infinitesrnal increment of such on process : SA + TdS = 0 ...
... T , we can write : Δ A + T Δ sirr = 0 For an infinitesrnal increment of such on process : SA + TdS = 0 ...
Solid - Liquid Phase Diagram of a Binary Mixture: The Question of
... derives from the fact that the quantity on the right depends solely on the temperature and properties of the pure component. It is important to note that as the activity of the component decreases (the Raoult’s Law activity cannot be greater than unity), the equilibrium temperature must decrease, si ...
... derives from the fact that the quantity on the right depends solely on the temperature and properties of the pure component. It is important to note that as the activity of the component decreases (the Raoult’s Law activity cannot be greater than unity), the equilibrium temperature must decrease, si ...
Equilibrium chemistry
Equilibrium chemistry is a concerned with systems in chemical equilibrium. The unifying principle is that the free energy of a system at equilibrium is the minimum possible, so that the slope of the free energy with respect to the reaction coordinate is zero. This principle, applied to mixtures at equilibrium provides a definition of an equilibrium constant. Applications include acid-base, host-guest, metal-complex, solubility, partition, chromatography and redox equilibria.