Thermochemistry Diploma Questions
... Novacor is a large international company that produces ethene (C2H4(g)) from ethane (C2H6(g)) at its plant near Joffre, Alberta. The essential process in the conversion of ethane to ethene is called cracking, which involves the removal of hydrogen atoms from ethane molecules. The cracking occurs in ...
... Novacor is a large international company that produces ethene (C2H4(g)) from ethane (C2H6(g)) at its plant near Joffre, Alberta. The essential process in the conversion of ethane to ethene is called cracking, which involves the removal of hydrogen atoms from ethane molecules. The cracking occurs in ...
Syllabus - Chemistry
... • Preparation of trans dicholoro bis (ethylenediamine) cobalt (III) chloride and its conversion to cis-isomer. • Preparation of tris (ethylenediamine) nickel (II) chloride dihydrate and its conversion to bis (ethylenediamine) nickel (II) chloride. • Preparation of bis (acetylacetonato) copper (II) d ...
... • Preparation of trans dicholoro bis (ethylenediamine) cobalt (III) chloride and its conversion to cis-isomer. • Preparation of tris (ethylenediamine) nickel (II) chloride dihydrate and its conversion to bis (ethylenediamine) nickel (II) chloride. • Preparation of bis (acetylacetonato) copper (II) d ...
2015 International Practice Exam: Chemistry
... sure infrared ports (Hewlett-Packard) are not facing each other . Since graphing calculators can be used to store data, including text, proctors should monitor that students are using their calculators appropriately. Attempts by students to use the calculator to remove exam questions and/or answers ...
... sure infrared ports (Hewlett-Packard) are not facing each other . Since graphing calculators can be used to store data, including text, proctors should monitor that students are using their calculators appropriately. Attempts by students to use the calculator to remove exam questions and/or answers ...
SUGGESTED TIMELINE: 4 Weeks - Hazlet Township Public Schools
... property of a substance that depends on the composition of a substance, not on the size of the sample. Density can be calculated by using the substance’s volume and mass. When a measurement is multiplied by a conversion factor, the numerical value and units are changed. However the quantity remains ...
... property of a substance that depends on the composition of a substance, not on the size of the sample. Density can be calculated by using the substance’s volume and mass. When a measurement is multiplied by a conversion factor, the numerical value and units are changed. However the quantity remains ...
Cl 2
... • Atoms – In formation of ammonia two atoms of nitrogen react with 6 atoms of hydrogen. These eight atoms are recombined in the product • Molecules – One molecule of nitrogen reacts with 3 molecules of hydrogen to form two molecules of ammonia • Moles – One mole of nitrogen gas reacts with 3 moles o ...
... • Atoms – In formation of ammonia two atoms of nitrogen react with 6 atoms of hydrogen. These eight atoms are recombined in the product • Molecules – One molecule of nitrogen reacts with 3 molecules of hydrogen to form two molecules of ammonia • Moles – One mole of nitrogen gas reacts with 3 moles o ...
AP Chemistry Notes and Worksheets 2014
... Dalton determined the first table of atomic weights. Many were wrong because of incorrect formulas. o Ex. OH for water with O having a mass of 8 and H having a mass of 1 Avogadro's Hypothesis- Gay Lussac and Avogadro studied the volumes of combining gases. This allowed them to determine correct ...
... Dalton determined the first table of atomic weights. Many were wrong because of incorrect formulas. o Ex. OH for water with O having a mass of 8 and H having a mass of 1 Avogadro's Hypothesis- Gay Lussac and Avogadro studied the volumes of combining gases. This allowed them to determine correct ...
Unit 13: Electrochemistry (Link to Prentice Hall Text: Chapters 22
... A car battery powers the car through a spontaneous reaction, but what can you do if the battery dies? (c) To coat one metal on top of another one, as with jewelry, or exhaust pipes. a. To make something look more expensive or shinier b. To improve corrosion resistance ...
... A car battery powers the car through a spontaneous reaction, but what can you do if the battery dies? (c) To coat one metal on top of another one, as with jewelry, or exhaust pipes. a. To make something look more expensive or shinier b. To improve corrosion resistance ...
chem A exercise package C
... electron into this overlapping region or into an electron "pool." By doing this, each atom appears to gain an electron within its original boundary. For every overlapping region an atom appears to gain one electron. Two overlapping regions, such as for oxygen, will result in the gain of two electron ...
... electron into this overlapping region or into an electron "pool." By doing this, each atom appears to gain an electron within its original boundary. For every overlapping region an atom appears to gain one electron. Two overlapping regions, such as for oxygen, will result in the gain of two electron ...
File - Junior College Chemistry tuition
... Gaseous particle U has an atomic number n and a charge of +1. Gaseous particle V has an atomic number of (n + 1) and is isoelectronic with U. Which of the following statements is always true? ...
... Gaseous particle U has an atomic number n and a charge of +1. Gaseous particle V has an atomic number of (n + 1) and is isoelectronic with U. Which of the following statements is always true? ...
Chemistry Senior External Syllabus 1998
... the senior school is divided into a number of disciplines, at the core of each lies continuous questioning of results and methods. Education in and through science is an avenue by which citizens may participate in its endeavours and influence the extent and direction of its use and application. As p ...
... the senior school is divided into a number of disciplines, at the core of each lies continuous questioning of results and methods. Education in and through science is an avenue by which citizens may participate in its endeavours and influence the extent and direction of its use and application. As p ...
Oxidation of benzoin with anchored vanadyl and
... alumina did catalyse the oxidation of benzoin to a very limited extent. With the anchored catalyst the reaction was faster. Thus, the oxidation of benzoin with all the polymer and alumina anchored catalysts using Bu’O,H oxidant was carried out under very similar conditions and the products analysed ...
... alumina did catalyse the oxidation of benzoin to a very limited extent. With the anchored catalyst the reaction was faster. Thus, the oxidation of benzoin with all the polymer and alumina anchored catalysts using Bu’O,H oxidant was carried out under very similar conditions and the products analysed ...
pH - OCCC.edu
... pH and pOH are related by the following equation that is derived by taking the negative log of the expression for Kw pH + pOH = 14.00 at 25oC ...
... pH and pOH are related by the following equation that is derived by taking the negative log of the expression for Kw pH + pOH = 14.00 at 25oC ...
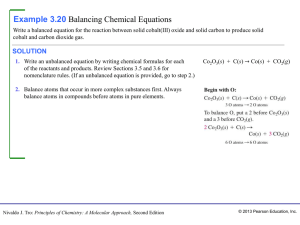
1. You should review balancing equations and identifying types of
... 1. You should review balancing equations and identifying types of reactions from the worksheets. In addition you should be able to write balanced chemical equations for reactions. Try to write, balance, and identify the types of the following reactions: a. the decomposition of ammonium nitrate to ni ...
... 1. You should review balancing equations and identifying types of reactions from the worksheets. In addition you should be able to write balanced chemical equations for reactions. Try to write, balance, and identify the types of the following reactions: a. the decomposition of ammonium nitrate to ni ...
Energy Changes in Chemical Reactions
... greater the thermal energy. The temperature3 of an object is a measure of its thermal energy content. Radiant energy4 is the energy carried by light, microwaves, and radio waves. Objects left in bright sunshine or exposed to microwaves become warm because much of the radiant energy they absorb is co ...
... greater the thermal energy. The temperature3 of an object is a measure of its thermal energy content. Radiant energy4 is the energy carried by light, microwaves, and radio waves. Objects left in bright sunshine or exposed to microwaves become warm because much of the radiant energy they absorb is co ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.
















![Neutral ionic liquid [BMIm]BF4 promoted highly selective](http://s1.studyres.com/store/data/017897985_1-047f9869d5604c115b21339541ccfffe-300x300.png)