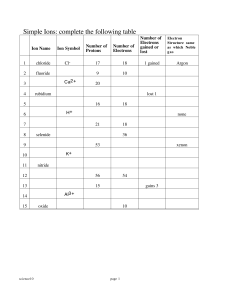
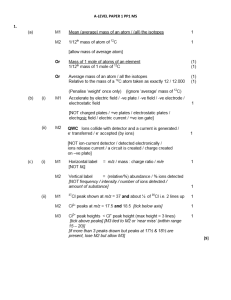
Sample Chapter - Chapter 4
... Recall from Section 2.7 that the electrons in a covalent bond are shared between the bonded atoms. In a covalent bond that exists between identical atoms (as in H2, Cl2, O2, etc.), the sharing is equal. As Figure 4.2A shows, the shared electrons in H2 are distributed equally, so no imbalance of char ...
... Recall from Section 2.7 that the electrons in a covalent bond are shared between the bonded atoms. In a covalent bond that exists between identical atoms (as in H2, Cl2, O2, etc.), the sharing is equal. As Figure 4.2A shows, the shared electrons in H2 are distributed equally, so no imbalance of char ...
workbook Chem (WP)
... Chemical vs Physical Properties 1. Identify the difference between a chemical and a physical change. ...
... Chemical vs Physical Properties 1. Identify the difference between a chemical and a physical change. ...
1 AM SYLLABUS (2016) CHEMISTRY AM 06 SYLLABUS
... Plot two variables from given data; understand that y = mx + c represents a linear relationship and be able to determine the slope and intercept of a line; draw and use the slope of a tangent to a curve as a measure of rate of change. ...
... Plot two variables from given data; understand that y = mx + c represents a linear relationship and be able to determine the slope and intercept of a line; draw and use the slope of a tangent to a curve as a measure of rate of change. ...
The SimSoup Guide - Chris Gordon
... possible are fixed, but the molecular structures and interactions are found as the simulation runs in a process of open-ended network discovery. ...
... possible are fixed, but the molecular structures and interactions are found as the simulation runs in a process of open-ended network discovery. ...
SQA CfE Higher Chemistry Unit 1: Chemical Changes and Structure
... Q3: A student obtains the following set of results shown in the graph when carrying out a reaction with marble chips and dilute hydrochloric acid. ...
... Q3: A student obtains the following set of results shown in the graph when carrying out a reaction with marble chips and dilute hydrochloric acid. ...
PHYSICAL SETTING CHEMISTRY
... 67 A spectral line in the infrared region of the spectrum of hydrogen has a frequency of 2.3 × 1014 hertz. Using your graph, estimate the energy associated with this spectral line. [1] 68 Explain, in terms of subatomic particles and energy states, why light is emitted by the hydrogen gas. [1] 69 Ide ...
... 67 A spectral line in the infrared region of the spectrum of hydrogen has a frequency of 2.3 × 1014 hertz. Using your graph, estimate the energy associated with this spectral line. [1] 68 Explain, in terms of subatomic particles and energy states, why light is emitted by the hydrogen gas. [1] 69 Ide ...
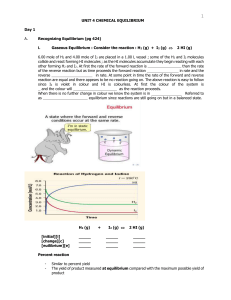
Unit 4 - Chemical Equilibrium
... 6.00 mole of H2 and 4.00 mole of I2 are placed in a 1.00 L vessel ; some of the H2 and I2 molecules collide and react forming HI molecules ; as the HI molecules accumulate they begin reacting with each other forming H2 and I2. At first the rate of the forward reaction is than the rate of the reverse ...
... 6.00 mole of H2 and 4.00 mole of I2 are placed in a 1.00 L vessel ; some of the H2 and I2 molecules collide and react forming HI molecules ; as the HI molecules accumulate they begin reacting with each other forming H2 and I2. At first the rate of the forward reaction is than the rate of the reverse ...
Introduction to Computational Chemistry Laboratory
... • Statistical Mechanics and Thermodynamics Statistical mechanics is the mathematical means to extrapolate thermodynamic properties of bulk materials from a molecular description of the material. Statistical mechanics computations are often tacked onto the end of ab inito calculations for gas phase p ...
... • Statistical Mechanics and Thermodynamics Statistical mechanics is the mathematical means to extrapolate thermodynamic properties of bulk materials from a molecular description of the material. Statistical mechanics computations are often tacked onto the end of ab inito calculations for gas phase p ...
Answers - University of Waterloo
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
... 5. Carefully detach the last page. It is the datasheet. 6. Now answer the exam questions. Questions are not in order of difficulty. Indicate your choice on the STUDENT RESPONSE sheet by marking one letter beside the question number. • Mark only one answer for each question. • Questions are all of th ...
Investigation of Atmospheric Pressure Plasma Source for CO Dissociation
... The CO can be filtered out for use in the chemical industry, or CO can be further combined in the plasma system with other gases such as hydrogen or water vapor to produce usable hydrocarbons. The design and use of a surface wave discharge excited by microwaves is experimentally examined here for th ...
... The CO can be filtered out for use in the chemical industry, or CO can be further combined in the plasma system with other gases such as hydrogen or water vapor to produce usable hydrocarbons. The design and use of a surface wave discharge excited by microwaves is experimentally examined here for th ...
440400 - IDEALS @ Illinois
... clouds of bound -molecular orbitals rather than with a discrete dipolar species. Specific systems: ...
... clouds of bound -molecular orbitals rather than with a discrete dipolar species. Specific systems: ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.