A Semi-Empirical Study on Metal Ion/Murexide
... as it is often extremely useful through is still not well understood. For example, the determination of rates of complexations of divalent metal ions with a tridentate metal-ion indicator dye murexide (Figure 1) in water are greatly affected by the addition of glycerol. This empirical technique of u ...
... as it is often extremely useful through is still not well understood. For example, the determination of rates of complexations of divalent metal ions with a tridentate metal-ion indicator dye murexide (Figure 1) in water are greatly affected by the addition of glycerol. This empirical technique of u ...
Chem. 31 * 9/15 Lecture
... “at equilibrium” conditions and K values or from initial conditions and K values – Be able to determine whether reaction will proceed to products or reactants from K and initial concentrations ...
... “at equilibrium” conditions and K values or from initial conditions and K values – Be able to determine whether reaction will proceed to products or reactants from K and initial concentrations ...
Integrated X-ray L Absorption Spectra. Counting Holes in Ni
... as an alternate scale for the ordinate in Figure 2 (right axis). Since different band structure calculations differ by ∼0.2 in the number of holes,54 the calculated values have an error on the order of 15-20%, and when ∼10% experimental errors are also considered, this intensity-to-3d holes conversi ...
... as an alternate scale for the ordinate in Figure 2 (right axis). Since different band structure calculations differ by ∼0.2 in the number of holes,54 the calculated values have an error on the order of 15-20%, and when ∼10% experimental errors are also considered, this intensity-to-3d holes conversi ...
2008 FALL Semester Midterm Examination For
... Please take a good use of the reference materials (Page 9 and 10), which include (a) Fundamental constants, (b) Conversion factors, and (c) Atomic weights of the elements. No questions are allowed during the exam. You are not allowed to leave during the exam. You have to hold your nature call. Pleas ...
... Please take a good use of the reference materials (Page 9 and 10), which include (a) Fundamental constants, (b) Conversion factors, and (c) Atomic weights of the elements. No questions are allowed during the exam. You are not allowed to leave during the exam. You have to hold your nature call. Pleas ...
Chapter 16
... A, B, C, and D that exist in equilibrium at a specified temperature and pressure. Let the number of moles of the respective components be NA, NB, NC, and ND. Now consider a reaction that occurs to an infinitesimal extent during which differential amounts of A and B (reactants) are converted to C and ...
... A, B, C, and D that exist in equilibrium at a specified temperature and pressure. Let the number of moles of the respective components be NA, NB, NC, and ND. Now consider a reaction that occurs to an infinitesimal extent during which differential amounts of A and B (reactants) are converted to C and ...
The solubility of Ca(OH) in extremely concentrated NaOH solutions
... is shown in Fig. 2. and in Table 1. The equilibrium concentration of Ca2+ steadily decreases with an increasing concentration of NaOH This indicates a lack of formation of higher, soluble, stepwise Ca(OH)n2–n hydroxo complexes (n > 2) even at the highest concentration of the base, as their formation ...
... is shown in Fig. 2. and in Table 1. The equilibrium concentration of Ca2+ steadily decreases with an increasing concentration of NaOH This indicates a lack of formation of higher, soluble, stepwise Ca(OH)n2–n hydroxo complexes (n > 2) even at the highest concentration of the base, as their formation ...
Chapter 23
... Chapter 23: The Chemical Bond in Diatomic Molecules Physical Chemistry 2nd Edition © 2010 Pearson Education South Asia Pte Ltd ...
... Chapter 23: The Chemical Bond in Diatomic Molecules Physical Chemistry 2nd Edition © 2010 Pearson Education South Asia Pte Ltd ...
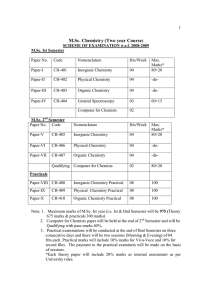
M.Sc. Chemistry (Two year Course)
... Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensional Rigid ro ...
... Schrodinger wave equation for a particle in a three dimensional box and the concept of degeneracy of energy levels. Schrodinger wave equation for linear harmonic oscillator, solution by polynomial method, zero point energy and its consequence. Schrodinger wave equation for three dimensional Rigid ro ...
- sartep.com
... Mass of the container plus the solid sample = 25.0 grams Volume of the solid sample = 11.0 cubic centimeters The data above were gathered in order to determine the density of an unknown solid. The density of the sample should be reported as: (A) 0.5 g/cm3 (B) 0.50 g/cm3 (C) 2.0 g/cm3 (D) 2.00 g/cm3 ...
... Mass of the container plus the solid sample = 25.0 grams Volume of the solid sample = 11.0 cubic centimeters The data above were gathered in order to determine the density of an unknown solid. The density of the sample should be reported as: (A) 0.5 g/cm3 (B) 0.50 g/cm3 (C) 2.0 g/cm3 (D) 2.00 g/cm3 ...
In situ-XAS and catalytic study of acrolein hydrogenation over silver
... been dried and reduced at 325 °C. The real metal content was 7.5% [6]. Additionally, the catalysts have been reduced in situ at 250 °C before each experiment in pure hydrogen. Mass flow controllers have been used for gas feeding; acrolein has been dosed by a liquid flow controller and evaporated aft ...
... been dried and reduced at 325 °C. The real metal content was 7.5% [6]. Additionally, the catalysts have been reduced in situ at 250 °C before each experiment in pure hydrogen. Mass flow controllers have been used for gas feeding; acrolein has been dosed by a liquid flow controller and evaporated aft ...
2.4 Chemical Reactions - Miami Beach Senior High School
... wood combine with oxygen from the air. • As the wood burns, a sizable amount of matter is reduced to a small pile of ashes. • The reaction seems to involve a reduction in the amount of matter. But appearances ...
... wood combine with oxygen from the air. • As the wood burns, a sizable amount of matter is reduced to a small pile of ashes. • The reaction seems to involve a reduction in the amount of matter. But appearances ...
Chemistry - Sanskriti School
... Unit XII: Organic Chemistry - Some Basic Principles and Techniques General introduction, methods of qualitative and quantitative analysis, classification and IUPAC . Nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and h ...
... Unit XII: Organic Chemistry - Some Basic Principles and Techniques General introduction, methods of qualitative and quantitative analysis, classification and IUPAC . Nomenclature of organic compounds. Electronic displacements in a covalent bond: inductive effect, electromeric effect, resonance and h ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.