Vinnitsa National Pirogov Memorial Medical University Biological
... What parts of chemistry did you study: inorganic chemistry yes/no, organic chemistry yes/no. After I had finished to study chemistry ______________ years/months passed. The students who studied chemistry at their countries we ask to read attentively the question given below. While answering the ques ...
... What parts of chemistry did you study: inorganic chemistry yes/no, organic chemistry yes/no. After I had finished to study chemistry ______________ years/months passed. The students who studied chemistry at their countries we ask to read attentively the question given below. While answering the ques ...
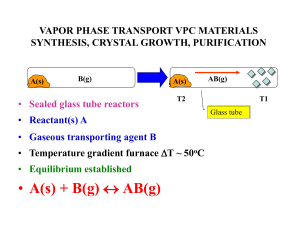
Chemical Reactions and Stoichiometry
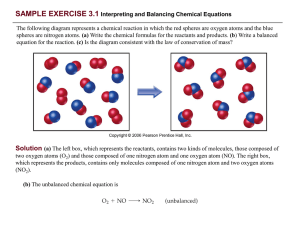
... The substances on the left side of the equation are called the reactants and the substances on the right side are called the products. We often specify the states of each reactant or product in parentheses next to the formula as follows: CH41g2 + O21g2 ¡ CO21g2 + H2O1g2 ...
... The substances on the left side of the equation are called the reactants and the substances on the right side are called the products. We often specify the states of each reactant or product in parentheses next to the formula as follows: CH41g2 + O21g2 ¡ CO21g2 + H2O1g2 ...
IChO_Comp_Prob_Answ 1997
... of the topics were felt to greatly exceed the knowledge base which a high school student, albeit even some of the best high school students in the world, could be reasonably expected to have without being exposed to rigorous and extensive additional study. It was strongly felt by some delegates that ...
... of the topics were felt to greatly exceed the knowledge base which a high school student, albeit even some of the best high school students in the world, could be reasonably expected to have without being exposed to rigorous and extensive additional study. It was strongly felt by some delegates that ...
CHEMISTRY Academic Standards Statement
... macroscopic properties of a chemical species. iv. The properties of a substance can be influenced by both physical and chemical environment. v. The properties of a mixture can differ from those of the individual components of the mixture. vi. Matter extends beyond the molecular to include metals, cr ...
... macroscopic properties of a chemical species. iv. The properties of a substance can be influenced by both physical and chemical environment. v. The properties of a mixture can differ from those of the individual components of the mixture. vi. Matter extends beyond the molecular to include metals, cr ...
PLACE LABEL HERE Tasmanian Certificate of Education
... Volume 1 may also be used throughout the examination. No other printed material will be allowed into the examination. An electronic calculator may be used. The examination consists of eight questions which are organised around the criteria 7, 8, 9 and 10. All questions should be answered. Answers mu ...
... Volume 1 may also be used throughout the examination. No other printed material will be allowed into the examination. An electronic calculator may be used. The examination consists of eight questions which are organised around the criteria 7, 8, 9 and 10. All questions should be answered. Answers mu ...
BASIC CONCEPTS OF CHEMISTRY
... Thus, the condition of the exothermic reaction is Q> 0 or H < 0, and the condition of the endothermic reaction - Q < 0 or H> 0 . The equation of a chemical reaction involving the magnitude of the thermal effect (enthalpy) is called a thermochemical equation: 2H2 ( g ) + O2 ( g ) = 2H2O ( g) + 571. ...
... Thus, the condition of the exothermic reaction is Q> 0 or H < 0, and the condition of the endothermic reaction - Q < 0 or H> 0 . The equation of a chemical reaction involving the magnitude of the thermal effect (enthalpy) is called a thermochemical equation: 2H2 ( g ) + O2 ( g ) = 2H2O ( g) + 571. ...
Module 1 Predictor Questions
... • In addition and subtraction problems, the final answer must contain no digits beyond the most doubtful digit in the numbers being added or subtracted. • In multiplication and division problems involving significant figures the final answer must contain the same number of significant figures as the ...
... • In addition and subtraction problems, the final answer must contain no digits beyond the most doubtful digit in the numbers being added or subtracted. • In multiplication and division problems involving significant figures the final answer must contain the same number of significant figures as the ...
The mole concept Since Compounds are formed
... a) You know the amount of one reactant b) You know the amount of at least two reactants E.g. a) How much magnesium oxide will form if 5.73 g magnesium burns in excess oxygen? 0) Think moles! 1) Convert mass of Mg to moles of Mg 5.73 g /24.31 g mol-1 = 2.36 x 10 -1 mol Mg 2) Convert moles of Mg to mo ...
... a) You know the amount of one reactant b) You know the amount of at least two reactants E.g. a) How much magnesium oxide will form if 5.73 g magnesium burns in excess oxygen? 0) Think moles! 1) Convert mass of Mg to moles of Mg 5.73 g /24.31 g mol-1 = 2.36 x 10 -1 mol Mg 2) Convert moles of Mg to mo ...
CHAPTER 19
... either atom has totally lost or totally gained any electrons. In the case of the formation of hydrogen chloride, for example, hydrogen simply has donated a share of its bonding electron to the chlorine; it has not completely transferred that electron. The assignment of oxidation numbers allows an ap ...
... either atom has totally lost or totally gained any electrons. In the case of the formation of hydrogen chloride, for example, hydrogen simply has donated a share of its bonding electron to the chlorine; it has not completely transferred that electron. The assignment of oxidation numbers allows an ap ...
14 - the Research Group of Angelika Kühnle
... In this review, basic principles behind molecular self-assembly of organic molecules on metal surfaces will be discussed. Controlling these formation principles allows for creating a wide variety of different molecular surface structures ranging from well-defined clusters, quasi onedimensional rows ...
... In this review, basic principles behind molecular self-assembly of organic molecules on metal surfaces will be discussed. Controlling these formation principles allows for creating a wide variety of different molecular surface structures ranging from well-defined clusters, quasi onedimensional rows ...
Table of Contents - slccscience`s Home Page
... alkali metals, alkali earth metals, chalcogens, halogens, and noble gases. Identify the three basic states of matter, the names of the changes between those states, and the role of temperature in the state in which matter is found. Describe how solids, liquids, and gases differ from each other at a ...
... alkali metals, alkali earth metals, chalcogens, halogens, and noble gases. Identify the three basic states of matter, the names of the changes between those states, and the role of temperature in the state in which matter is found. Describe how solids, liquids, and gases differ from each other at a ...
analytical chemistry - Львівський національний медичний
... In all of classifications identical is the group of cations, which sediment by sulphate acid, ammonium carbonate, and sodium hydrogenphosphate in ammonia presence. There are the cations of the s2 elements: calcium, barium, and strontium, which are found in ІІАsub-group of periodic system. Precipitat ...
... In all of classifications identical is the group of cations, which sediment by sulphate acid, ammonium carbonate, and sodium hydrogenphosphate in ammonia presence. There are the cations of the s2 elements: calcium, barium, and strontium, which are found in ІІАsub-group of periodic system. Precipitat ...
Chapter 12
... the amounts of reactants used and the amounts of products formed by a chemical reaction. What are the tools needed for stoichiometric calculations? All stoichiometric calculations begin with a balanced chemical equation, which indicates relative amounts of the substances that react and the products ...
... the amounts of reactants used and the amounts of products formed by a chemical reaction. What are the tools needed for stoichiometric calculations? All stoichiometric calculations begin with a balanced chemical equation, which indicates relative amounts of the substances that react and the products ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.