CHEM-1411 Final Practice Exam
... Since there are a total of four atoms plus lone pairs (four “electron domains”) around the central sulfur, the overall geometry is tetrahedral and the molecular geometry is trigonal pyramidal. The hybridization of the sulfur atom in the first structure is therefore sp3. However, the sulfur is not s ...
... Since there are a total of four atoms plus lone pairs (four “electron domains”) around the central sulfur, the overall geometry is tetrahedral and the molecular geometry is trigonal pyramidal. The hybridization of the sulfur atom in the first structure is therefore sp3. However, the sulfur is not s ...
- StarBooks
... Zeros at the beginning of a number are not significant. For example, 0.002 has one significant figure while 0.0045has two significant figures. All zeros placed to the right of a number are significant. For example, 16.0 has three significant figures, while 16.00has four significant figures. Zeros at ...
... Zeros at the beginning of a number are not significant. For example, 0.002 has one significant figure while 0.0045has two significant figures. All zeros placed to the right of a number are significant. For example, 16.0 has three significant figures, while 16.00has four significant figures. Zeros at ...
Detailed modeling of the evaporation and thermal decomposition of
... In the present work, a UWS droplet evaporation model is proposed, based on the multicomponent droplet evaporation model developed by the authors [10]. The suggested analysis is based on the conventional conservation equations of species and energy for the gas phase, and the energy balance equation a ...
... In the present work, a UWS droplet evaporation model is proposed, based on the multicomponent droplet evaporation model developed by the authors [10]. The suggested analysis is based on the conventional conservation equations of species and energy for the gas phase, and the energy balance equation a ...
Chemistry
... a) Give steps (using correct terms for glassware, chemicals, and lab equipment) for the experiment you need to perform in order to determine the concentration of the HCl. b) What is this type of experiment called? c) Indicate what measurements need to be taken (you do not have to discuss any calcula ...
... a) Give steps (using correct terms for glassware, chemicals, and lab equipment) for the experiment you need to perform in order to determine the concentration of the HCl. b) What is this type of experiment called? c) Indicate what measurements need to be taken (you do not have to discuss any calcula ...
kinetic characterisation of catalysts for methanol synthesis
... activity and durability an interest to develop better catalyst has steadily been observed. In order to improve the efficiency of the process, modified catalysts are examined. The Cu/ZnO/ZrO2 catalyst and the addition of B, Ga, In, Gd, Y, Mn and Mg oxides were studied by Skrzypek et al. (2006). The a ...
... activity and durability an interest to develop better catalyst has steadily been observed. In order to improve the efficiency of the process, modified catalysts are examined. The Cu/ZnO/ZrO2 catalyst and the addition of B, Ga, In, Gd, Y, Mn and Mg oxides were studied by Skrzypek et al. (2006). The a ...
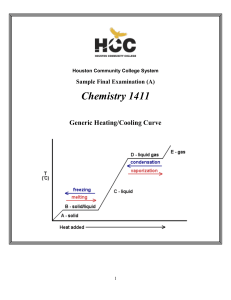
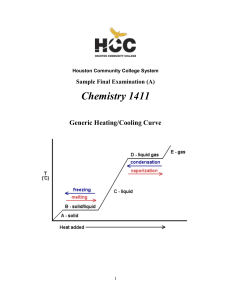
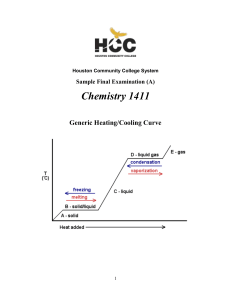
Thermodynamics: the Second Law
... doing work on the refrigerator, then the entropy change of the warm exterior can be increased to the point at which it overcomes the decrease in entropy of the cold interior, and the refrigerator operates. The calculation of the maximum efficiency of this process is left as an exercise (see Project ...
... doing work on the refrigerator, then the entropy change of the warm exterior can be increased to the point at which it overcomes the decrease in entropy of the cold interior, and the refrigerator operates. The calculation of the maximum efficiency of this process is left as an exercise (see Project ...
Chapter 23 + Practice Problems - Bloomsburg Area School District
... Proteins are found in all living cells and are the most complex and varied class of biochemical molecules. A protein is an organic biological polymer that is made up of polypeptide chains of 50 or more amino acids and is an important building block of all cells. The name protein comes from the Greek ...
... Proteins are found in all living cells and are the most complex and varied class of biochemical molecules. A protein is an organic biological polymer that is made up of polypeptide chains of 50 or more amino acids and is an important building block of all cells. The name protein comes from the Greek ...
17.2 The Avogadro Number
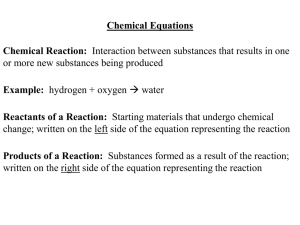
... Chemical equations show what is happening in a chemical reaction. They provide you with the identities of the reactants (substances entering the reaction) and the products (substances formed by the reaction). They also tell you how much of each substance is involved in the reaction. Chemical equatio ...
... Chemical equations show what is happening in a chemical reaction. They provide you with the identities of the reactants (substances entering the reaction) and the products (substances formed by the reaction). They also tell you how much of each substance is involved in the reaction. Chemical equatio ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.