Physics, Biology
... process of observation and experimentation, Galileo Galilei used experiments to overturn Aristotle’s ideas of the motion of objects, for example the flawed idea that heavy objects fall faster than lighter ones, which dominated physics for about 2000 years. The greatest contribution to the developmen ...
... process of observation and experimentation, Galileo Galilei used experiments to overturn Aristotle’s ideas of the motion of objects, for example the flawed idea that heavy objects fall faster than lighter ones, which dominated physics for about 2000 years. The greatest contribution to the developmen ...
Computational investigations of the electronic structure of molecular
... well as the continuous support in my research and being a fellow Swede abroad. Still need to work a bit on the nationalism but overall a very good friend. I would also like to thank Rosie, Luke, Kieran, Amy, Andrea, German, Andy, Ross, Zoso, Matt, Laura and everyone, past and present, who has been w ...
... well as the continuous support in my research and being a fellow Swede abroad. Still need to work a bit on the nationalism but overall a very good friend. I would also like to thank Rosie, Luke, Kieran, Amy, Andrea, German, Andy, Ross, Zoso, Matt, Laura and everyone, past and present, who has been w ...
Chap 3 - HCC Learning Web
... Since C4H10 contains 4 carbon atoms, so we need four carbon atoms at the right side, which leads us to put 4 (called coefficient) in front of the CO2. Now the equation is updated to be 1 C4H10 + __ O2 4 CO2 + __ H2O As there are 10 hydrogen atoms in C4H10, thus we need to balance the hydrogen atom ...
... Since C4H10 contains 4 carbon atoms, so we need four carbon atoms at the right side, which leads us to put 4 (called coefficient) in front of the CO2. Now the equation is updated to be 1 C4H10 + __ O2 4 CO2 + __ H2O As there are 10 hydrogen atoms in C4H10, thus we need to balance the hydrogen atom ...
Answers - Pearson
... value for average kinetic energy.) 7 From the kinetic molecular theory we would expect a solid to be more dense than its liquid, and therefore that ice would sink in water. 8 Bubbles will be present through the volume of the liquid. A brown gas is visible above the brown liquid. As the two state ...
... value for average kinetic energy.) 7 From the kinetic molecular theory we would expect a solid to be more dense than its liquid, and therefore that ice would sink in water. 8 Bubbles will be present through the volume of the liquid. A brown gas is visible above the brown liquid. As the two state ...
Module 3: Defects, Diffusion and Conduction in Ceramics
... called as diffusion. Diffusivity of species in the materials is also related to their physical properties such as electrical conductivity and mobility via Nernst-Einestein relation which we shall derive. As we shall also see, the conductivity in ceramics is a sum of ionic and electronic conductivity ...
... called as diffusion. Diffusivity of species in the materials is also related to their physical properties such as electrical conductivity and mobility via Nernst-Einestein relation which we shall derive. As we shall also see, the conductivity in ceramics is a sum of ionic and electronic conductivity ...
Chapter 10
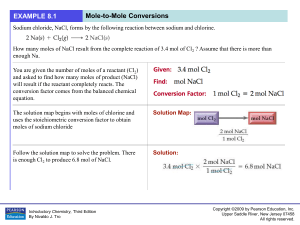
... Chapter Summary The coefficients in a balanced chemical reaction are the mole ratio of the reactants and products. The coefficients in a balanced chemical reaction are the volume ratio of gaseous reactants and products. We can convert moles or liters of a given substance to moles or liters of ...
... Chapter Summary The coefficients in a balanced chemical reaction are the mole ratio of the reactants and products. The coefficients in a balanced chemical reaction are the volume ratio of gaseous reactants and products. We can convert moles or liters of a given substance to moles or liters of ...
Post Lab Questions
... then worst three will be dropped). Some quizzes will be given without prior planning. Problem sets will be assigned frequently. They will be checked in class on the following day. They may or may not be collected for credit. Laboratory reports are due the day after the lab is completed. The only exc ...
... then worst three will be dropped). Some quizzes will be given without prior planning. Problem sets will be assigned frequently. They will be checked in class on the following day. They may or may not be collected for credit. Laboratory reports are due the day after the lab is completed. The only exc ...
LABORATORY MANUAL FOR GENERAL CHEMISTRY I
... reasons, your lab instructor is present to assist you. He is your friend. Treat him well and above all don’t be afraid to ask him questions. Within reason, he will be glad to help you. Chemistry is an experimental science. The knowledge that has been accumulated through previous experiments provides ...
... reasons, your lab instructor is present to assist you. He is your friend. Treat him well and above all don’t be afraid to ask him questions. Within reason, he will be glad to help you. Chemistry is an experimental science. The knowledge that has been accumulated through previous experiments provides ...
Zero-Field Splitting in Transition Metal Complexes: Ab Initio
... complexes. In this case, the “full” set of spin-orbit free states consists of ten spinquartet (10Q) and 40 spin-doublet (40D) SOF states, while it is also safe to consider the 4Q and 7Q subsets [44]. Now that the set of SOF states has been defined, the SOC is computed between the spin components (i. ...
... complexes. In this case, the “full” set of spin-orbit free states consists of ten spinquartet (10Q) and 40 spin-doublet (40D) SOF states, while it is also safe to consider the 4Q and 7Q subsets [44]. Now that the set of SOF states has been defined, the SOC is computed between the spin components (i. ...
Antimony-ligated dysprosium single-molecule magnets as catalysts
... magnets as catalysts for stibine dehydrocoupling† Thomas Pugh, Nicholas F. Chilton* and Richard A. Layfield* Single-molecule magnets (SMMs) are coordination compounds that exhibit magnetic bistability below a characteristic blocking temperature. Research in this field continues to evolve from its fund ...
... magnets as catalysts for stibine dehydrocoupling† Thomas Pugh, Nicholas F. Chilton* and Richard A. Layfield* Single-molecule magnets (SMMs) are coordination compounds that exhibit magnetic bistability below a characteristic blocking temperature. Research in this field continues to evolve from its fund ...
B.Sc. Industrial Chemistry
... and monitoring of various chemical processes used in industry for transforming raw materials etc., into useful commercial products for society. Industrial chemistry as an applied science plays a vital role in diverse areas that influence human society. The course in the present form focuses on indus ...
... and monitoring of various chemical processes used in industry for transforming raw materials etc., into useful commercial products for society. Industrial chemistry as an applied science plays a vital role in diverse areas that influence human society. The course in the present form focuses on indus ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.