std 8 9 reviewanswers
... A The larger the amount of activation energy, the more unstable the transition state is B The activation energy is the difference energy between the products and the transition state C A catalyst works by lowering the activation energy for the reaction 9. Chemical equilibrium is a dynamic process at ...
... A The larger the amount of activation energy, the more unstable the transition state is B The activation energy is the difference energy between the products and the transition state C A catalyst works by lowering the activation energy for the reaction 9. Chemical equilibrium is a dynamic process at ...
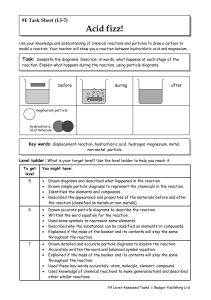
Year 9 Homework Task 9E-5 Reactions 5-7
... Drawn detailed and accurate particle diagrams to explain the reaction. Accurately written the word and balanced symbol equation. Explained if the mass of the beaker and its contents will stay the same throughout the reaction. Used these key words accurately: atom, molecule, element, compound. Used k ...
... Drawn detailed and accurate particle diagrams to explain the reaction. Accurately written the word and balanced symbol equation. Explained if the mass of the beaker and its contents will stay the same throughout the reaction. Used these key words accurately: atom, molecule, element, compound. Used k ...
CHM_101_ASSIGNMENT_COPY_1_2
... 1. Calculate the change in PH obtained on the addition of 0.03 mole of solid NaOH to a buffer solution that consists of 0.15M sodium acetate and 0.15M acetic acid solution, if we assume that there is no change in volume (Ka = 1.8 x 10-5). 2. (a) The rate constant of a first order reaction is 2.5 ×1 ...
... 1. Calculate the change in PH obtained on the addition of 0.03 mole of solid NaOH to a buffer solution that consists of 0.15M sodium acetate and 0.15M acetic acid solution, if we assume that there is no change in volume (Ka = 1.8 x 10-5). 2. (a) The rate constant of a first order reaction is 2.5 ×1 ...
OBL - USM
... Derive ideal gas law from the kinetic theory of gases. Apply the Maxwell-Boltzmann equation to calculate the probability of the gas molecules (to determine the number/fraction of molecules) between two molecular speeds. Distinguish the different molecular speeds. Calculate the three different molecu ...
... Derive ideal gas law from the kinetic theory of gases. Apply the Maxwell-Boltzmann equation to calculate the probability of the gas molecules (to determine the number/fraction of molecules) between two molecular speeds. Distinguish the different molecular speeds. Calculate the three different molecu ...
Chapter 2: Chemical Reactions Section 1
... reactants & products ID element that is not equal on both sides Add coefficient to the front of the formula that will make the # = on both sides for that element ...
... reactants & products ID element that is not equal on both sides Add coefficient to the front of the formula that will make the # = on both sides for that element ...
File
... _____ 14. The activity series of metals can be used to predict products in double-replacement reactions. _____ 15. Carbon dioxide and water are the products of the combustion of hexane (C6H14). _____ 16. A nonmetal can replace another nonmetal from a compound in a single-replacement reaction. ...
... _____ 14. The activity series of metals can be used to predict products in double-replacement reactions. _____ 15. Carbon dioxide and water are the products of the combustion of hexane (C6H14). _____ 16. A nonmetal can replace another nonmetal from a compound in a single-replacement reaction. ...
Week - Mat-Su School District
... i. Acid Base 1. Arrhenius 2. Bronstead Lowry 3. Lewis ii. Precipitation iii. Oxidation-reduction (redox) 1. Oxidation numbers 2. The electrons role 3. Electrochemistry, electrolytic & galvanic cells, Faradays laws, standard half-cell potentials, Nernst equation ...
... i. Acid Base 1. Arrhenius 2. Bronstead Lowry 3. Lewis ii. Precipitation iii. Oxidation-reduction (redox) 1. Oxidation numbers 2. The electrons role 3. Electrochemistry, electrolytic & galvanic cells, Faradays laws, standard half-cell potentials, Nernst equation ...
physics/0010052 PDF
... Van’t-Hoff equation it is necessary to take into account the law of conservation in the form of (6), not of (2). If to derive the Van’t-Hoff equation taking into account (6) the result will be the following one: d/dT ln K=∆H*0/RT2 ...
... Van’t-Hoff equation it is necessary to take into account the law of conservation in the form of (6), not of (2). If to derive the Van’t-Hoff equation taking into account (6) the result will be the following one: d/dT ln K=∆H*0/RT2 ...
Faculty of Science Department of chemistry Physical Chemistry (2)
... Apply kinetic treatments to complex reactions: chain, explosion, polymerization, and autocatalytic reaction. ...
... Apply kinetic treatments to complex reactions: chain, explosion, polymerization, and autocatalytic reaction. ...
Chapter 7
... temperature will increase the reaction rate. • Ex: You store milk in a refrigerator to slow down the reactions that cause the milk to spoil • Increasing the temperature of a substance causes its particles to move faster, on average. • Particles that move faster are both more likely to collide and mo ...
... temperature will increase the reaction rate. • Ex: You store milk in a refrigerator to slow down the reactions that cause the milk to spoil • Increasing the temperature of a substance causes its particles to move faster, on average. • Particles that move faster are both more likely to collide and mo ...
Lecture 4. - ChemWeb (UCC)
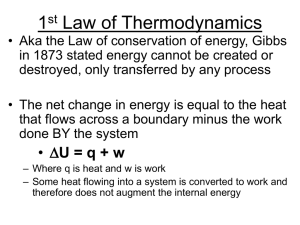
... • Energy associated with random motion of atoms and molecules Chemical Energy • Energy stored in structural units of chemicals Units = Joules • 1 Joule (J) = 1 kg m2 /s2 • 1 Calorie (cal) = 4.184 J ...
... • Energy associated with random motion of atoms and molecules Chemical Energy • Energy stored in structural units of chemicals Units = Joules • 1 Joule (J) = 1 kg m2 /s2 • 1 Calorie (cal) = 4.184 J ...
Standard answers: 1 Basic concepts, Fuels, alkanes and alkenes
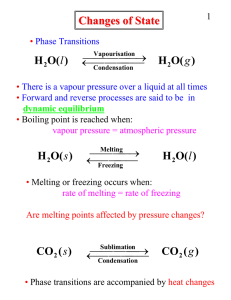
... 34. Features of an equilibrium Closed system Dynamic 35. What can be said about the forward and reverse reactions at equilibrium The rate of the forward and reverse reactions are the same 36. Effect of pressure on equilibrium Increasing P moves the eqm to the side with fewer moles of gas (an ...
... 34. Features of an equilibrium Closed system Dynamic 35. What can be said about the forward and reverse reactions at equilibrium The rate of the forward and reverse reactions are the same 36. Effect of pressure on equilibrium Increasing P moves the eqm to the side with fewer moles of gas (an ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.