Please do not remove this page. The periodic table, constants, and
... For a reversible exothermic reaction, the activation energy for the forward reaction is __________________ the activation energy of the reverse reaction. Which one answer below best fills in this blank to complete the statement? less than ...
... For a reversible exothermic reaction, the activation energy for the forward reaction is __________________ the activation energy of the reverse reaction. Which one answer below best fills in this blank to complete the statement? less than ...
AP CHEMISTRY SYLLABUS STUDENT VERSION
... arrangements of these atoms. These atoms retain their identity in chemical reactions. Big Idea 2- Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ions, and molecules and the forces between them. Big Idea 3- Changes in matter involve the r ...
... arrangements of these atoms. These atoms retain their identity in chemical reactions. Big Idea 2- Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ions, and molecules and the forces between them. Big Idea 3- Changes in matter involve the r ...
Exam Review Chapter 18-Equilibrium
... 6. What is the effect of adding more CO2 to the following equilibrium reaction? CO2 + H2O↔ H2CO3 a. More H2CO3 is produced. b. More H2O is produced. c. The equilibrium d. No Change 7. Two opposing reactions (A + B ↔C + D) occurring simultaneously at the same rate is an example of: a. reversibility. ...
... 6. What is the effect of adding more CO2 to the following equilibrium reaction? CO2 + H2O↔ H2CO3 a. More H2CO3 is produced. b. More H2O is produced. c. The equilibrium d. No Change 7. Two opposing reactions (A + B ↔C + D) occurring simultaneously at the same rate is an example of: a. reversibility. ...
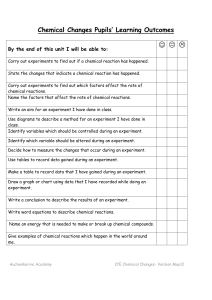
Chemical Reactions Unit Pupils` Learning Outcomes
... By the end of this unit I will be able to: Carry out experiments to find out if a chemical reaction has happened. State the changes that indicate a chemical reaction has happened. Carry out experiments to find out which factors effect the rate of chemical reactions. Name the factors that affect the ...
... By the end of this unit I will be able to: Carry out experiments to find out if a chemical reaction has happened. State the changes that indicate a chemical reaction has happened. Carry out experiments to find out which factors effect the rate of chemical reactions. Name the factors that affect the ...
- Lexington JHS
... Elements-found on the periodic table An element is a pure substance that cannot be broken down into other substances. (made of only one type of atom) Ex. C, H, O, S, or Fe ...
... Elements-found on the periodic table An element is a pure substance that cannot be broken down into other substances. (made of only one type of atom) Ex. C, H, O, S, or Fe ...
Chemical Synthesis (sat6)
... A1: MgO and H2 -> Mg and H2O; A2: C and O2 -> CO2; A3: CO2 and H2O -> H2CO3; A4: MgO and H2 and O2 and C; minimize obj: H2CO3; Write(’Yes, H2CO3 is produced’); Write(’No, H2CO3 is not produced’); ...
... A1: MgO and H2 -> Mg and H2O; A2: C and O2 -> CO2; A3: CO2 and H2O -> H2CO3; A4: MgO and H2 and O2 and C; minimize obj: H2CO3; Write(’Yes, H2CO3 is produced’); Write(’No, H2CO3 is not produced’); ...
system = part of the universe that contains the reaction or process
... (energy!) that accompany chemical reactions and phase changes • Heat is exchanged between the system and surroundings ...
... (energy!) that accompany chemical reactions and phase changes • Heat is exchanged between the system and surroundings ...
Section 1 Atoms, Elements, and Compounds
... o Mass is never created or destroyed. Some you will have the same elements on both sides of an equation. Balancing o The number of atoms must be the same on both sides of an equation. ...
... o Mass is never created or destroyed. Some you will have the same elements on both sides of an equation. Balancing o The number of atoms must be the same on both sides of an equation. ...
Lecture #5
... Molecularity: The number of molecules in the activated complex. Thermodynamics tells only that Ea > H. III D. KINETIC THEORY OF GASES When molecules in the gas phase collide they sometimes rearrange their chemical bonds to form new molecules. The rate of formation of the new molecules is determined ...
... Molecularity: The number of molecules in the activated complex. Thermodynamics tells only that Ea > H. III D. KINETIC THEORY OF GASES When molecules in the gas phase collide they sometimes rearrange their chemical bonds to form new molecules. The rate of formation of the new molecules is determined ...
Chemical Reactions - Mr. Brown`s Science Town
... An Intro. To Chemical Reactions What is a chemical reaction? How do we represent chemical reactions? ...
... An Intro. To Chemical Reactions What is a chemical reaction? How do we represent chemical reactions? ...
Chemistry CP Final Exam Review #2
... 1. The temperature of a metal bar with a mass of 87 grams is raised from 31oC to 132oC. In the process, 790.0 Joules of heat were absorbed. Find the specific heat of the metal. ...
... 1. The temperature of a metal bar with a mass of 87 grams is raised from 31oC to 132oC. In the process, 790.0 Joules of heat were absorbed. Find the specific heat of the metal. ...
document
... 1. At a transition temperature (Tf or Tb), the temperature remains constant until the phase change is complete 2. At the transition temperature, the transfer of heat is reversible 3. Because we are at constant pressure, the heat supplied is equal to the ...
... 1. At a transition temperature (Tf or Tb), the temperature remains constant until the phase change is complete 2. At the transition temperature, the transfer of heat is reversible 3. Because we are at constant pressure, the heat supplied is equal to the ...
Oxidation-Reduction Reactions
... The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds, where it is -1, eg. LiH Fluorine is –1 in all its compounds. Each of the other halogens is –1 in binary compounds unless the other element is oxygen. The sum of the oxidation numbers of the atoms in a comp ...
... The oxidation number of hydrogen is +1 except when it is bonded to metals in binary compounds, where it is -1, eg. LiH Fluorine is –1 in all its compounds. Each of the other halogens is –1 in binary compounds unless the other element is oxygen. The sum of the oxidation numbers of the atoms in a comp ...
Chemical reactions
... reaction. They are written in the left term of the equation. Reaction products = substances formed in a chemical reaction. They are written in the right term of the equation Because in a chemical reaction, the nature of atoms of the substances is not changed, the chemical equations are equalized so ...
... reaction. They are written in the left term of the equation. Reaction products = substances formed in a chemical reaction. They are written in the right term of the equation Because in a chemical reaction, the nature of atoms of the substances is not changed, the chemical equations are equalized so ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.