Unit 1 - Physical Chemistry REACTION KINETICS
... The rate determing step would be step ① as it is the slow step and only chemicals involved in the rate determining step would effect the rate of reaction. HI is a reaction intermediate and would be used up as fast as it is produced. Proving a mechanism can be very difficult. Detecting intermediates ...
... The rate determing step would be step ① as it is the slow step and only chemicals involved in the rate determining step would effect the rate of reaction. HI is a reaction intermediate and would be used up as fast as it is produced. Proving a mechanism can be very difficult. Detecting intermediates ...
lec03 - McMaster Chemistry
... In a chemical reaction: • if more energy is released in forming bonds than is used in breaking bonds then . . . reaction is EXOTHERMIC Energy is released as HEAT, LIGHT, SOUND, WORK • if more energy is used in breaking bonds than is released in forming bonds then . . . reaction is ENDOTHERMIC ...
... In a chemical reaction: • if more energy is released in forming bonds than is used in breaking bonds then . . . reaction is EXOTHERMIC Energy is released as HEAT, LIGHT, SOUND, WORK • if more energy is used in breaking bonds than is released in forming bonds then . . . reaction is ENDOTHERMIC ...
1.8 Thermodynamics
... The entropy contribution depends on temperature, T (K) at which the reaction takes place. TDS ...
... The entropy contribution depends on temperature, T (K) at which the reaction takes place. TDS ...
chemistry
... be done in pencil. You may use scrap paper to work out the answers to the questions, but be sure to record all your answers in your answer booklet. When you have completed the examination, you must sign the statement printed on the first page of your answer booklet, indicating that you had no unlawf ...
... be done in pencil. You may use scrap paper to work out the answers to the questions, but be sure to record all your answers in your answer booklet. When you have completed the examination, you must sign the statement printed on the first page of your answer booklet, indicating that you had no unlawf ...
View PDF
... a. A is the limiting reactant. c. there is no limiting reactant. b. B is the limiting reactant. d. no product can be formed. ____ 18. What is the maximum possible amount of product obtained in a chemical reaction? a. theoretical yield c. mole ratio b. percent yield d. actual yield ____ 19. A chemist ...
... a. A is the limiting reactant. c. there is no limiting reactant. b. B is the limiting reactant. d. no product can be formed. ____ 18. What is the maximum possible amount of product obtained in a chemical reaction? a. theoretical yield c. mole ratio b. percent yield d. actual yield ____ 19. A chemist ...
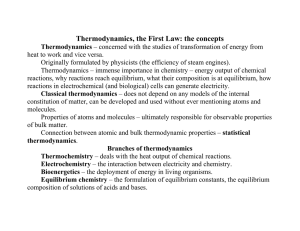
The basic concepts and Thermochemistry
... Because the potential energy of interactions between the atoms or molecules of a perfect gas is zero, the internal energy is independent of how close they are together, i.e., of volume: ∂U = 0 . The internal energy of interacting molecules in addition has a contribution from ∂V T the pote ...
... Because the potential energy of interactions between the atoms or molecules of a perfect gas is zero, the internal energy is independent of how close they are together, i.e., of volume: ∂U = 0 . The internal energy of interacting molecules in addition has a contribution from ∂V T the pote ...
File
... Atoms want their valence shell to be full so they gain or lose electrons. Once the atom no longer has the same number of electrons as protons the atom has a charge. It is now called an ion instead. o Formation of Ions: Positive Ions are called CATIONS – they have lost electrons (metals do this) ...
... Atoms want their valence shell to be full so they gain or lose electrons. Once the atom no longer has the same number of electrons as protons the atom has a charge. It is now called an ion instead. o Formation of Ions: Positive Ions are called CATIONS – they have lost electrons (metals do this) ...
Equilibrium and Pressure
... Introduction: In the Equilibrium and Concentration Gizmo, you found that reversible reactions eventually result in chemical equilibrium. Chemical equilibrium is reached when the rates of the forward and reverse reactions are the same. The constant Kc describes the ratio of products to reactants at e ...
... Introduction: In the Equilibrium and Concentration Gizmo, you found that reversible reactions eventually result in chemical equilibrium. Chemical equilibrium is reached when the rates of the forward and reverse reactions are the same. The constant Kc describes the ratio of products to reactants at e ...
Chemistry Note PowerPoint
... • Reactants = substances that you start with at the beginning of a reaction • Products = substances that you end with after a reaction has occurred • Subscript = the number in a chemical formula that tells the number of atoms in a molecule • Coefficient = is the number placed in front of a chemical ...
... • Reactants = substances that you start with at the beginning of a reaction • Products = substances that you end with after a reaction has occurred • Subscript = the number in a chemical formula that tells the number of atoms in a molecule • Coefficient = is the number placed in front of a chemical ...
Focus 4A-F
... Practice 7: Suppose that 1.00 mol of ideal gas molecules at 292 K and 3.00 atm expands from 8.00 L to 20.00 L and a final pressure of 1.20 atm by two different paths. (a) Path A is an isothermal, reversible expansion. (b) Path B has two parts. In step 1, the gas is cooled at constant volume until i ...
... Practice 7: Suppose that 1.00 mol of ideal gas molecules at 292 K and 3.00 atm expands from 8.00 L to 20.00 L and a final pressure of 1.20 atm by two different paths. (a) Path A is an isothermal, reversible expansion. (b) Path B has two parts. In step 1, the gas is cooled at constant volume until i ...
Understanding the Role of Aqueous Solution in Chemical Reactions
... Due to its properties, solvent effects in aqueous chemical reactions are often significant. Both beneficial and negative effects of water in chemical reactions are well documented. Compared to the organic alternatives, water is an environmentally friendly, cheap and safe solvent. To date, the role o ...
... Due to its properties, solvent effects in aqueous chemical reactions are often significant. Both beneficial and negative effects of water in chemical reactions are well documented. Compared to the organic alternatives, water is an environmentally friendly, cheap and safe solvent. To date, the role o ...
www.xtremepapers.net
... A study was carried out in which both [H2O2] and [H+] were kept constant at 0.05 mol dm–3, and [I–] was plotted against time. The following curve was obtained. ...
... A study was carried out in which both [H2O2] and [H+] were kept constant at 0.05 mol dm–3, and [I–] was plotted against time. The following curve was obtained. ...
Transition state theory
Transition state theory (TST) explains the reaction rates of elementary chemical reactions. The theory assumes a special type of chemical equilibrium (quasi-equilibrium) between reactants and activated transition state complexes.TST is used primarily to understand qualitatively how chemical reactions take place. TST has been less successful in its original goal of calculating absolute reaction rate constants because the calculation of absolute reaction rates requires precise knowledge of potential energy surfaces, but it has been successful in calculating the standard enthalpy of activation (Δ‡Hɵ), the standard entropy of activation (Δ‡Sɵ), and the standard Gibbs energy of activation (Δ‡Gɵ) for a particular reaction if its rate constant has been experimentally determined. (The ‡ notation refers to the value of interest at the transition state.)This theory was developed simultaneously in 1935 by Henry Eyring, then at Princeton University, and by Meredith Gwynne Evans and Michael Polanyi of the University of Manchester. TST is also referred to as ""activated-complex theory,"" ""absolute-rate theory,"" and ""theory of absolute reaction rates.""Before the development of TST, the Arrhenius rate law was widely used to determine energies for the reaction barrier. The Arrhenius equation derives from empirical observations and ignores any mechanistic considerations, such as whether one or more reactive intermediates are involved in the conversion of a reactant to a product. Therefore, further development was necessary to understand the two parameters associated with this law, the pre-exponential factor (A) and the activation energy (Ea). TST, which led to the Eyring equation, successfully addresses these two issues; however, 46 years elapsed between the publication of the Arrhenius rate law, in 1889, and the Eyring equation derived from TST, in 1935. During that period, many scientists and researchers contributed significantly to the development of the theory.