Chapter 21: Optical Properties
... • Apply strong forward bias across semiconductor layers, metal, and heat sink. • Electron-hole pairs generated by electrons that are excited across band gap. • Recombination of an electron-hole pair generates a photon of laser light ...
... • Apply strong forward bias across semiconductor layers, metal, and heat sink. • Electron-hole pairs generated by electrons that are excited across band gap. • Recombination of an electron-hole pair generates a photon of laser light ...
What is a mixture?
... Identifying Elements • Elements are categorized by unique properties on the Periodic Table. • They are arranged in order by their number of protons. (More on this later!) • Each element has unique properties like melting point, boiling point, and whether it is metal, nonmetal or metalloid. ...
... Identifying Elements • Elements are categorized by unique properties on the Periodic Table. • They are arranged in order by their number of protons. (More on this later!) • Each element has unique properties like melting point, boiling point, and whether it is metal, nonmetal or metalloid. ...
Solve the following problems using the intensity equation
... 1. A light ray is an imaginary line that represents a thin beam of light in a ray diagram. Drawing light rays is helpful in predicting the location and size of an image that will be created by a mirror or lens. 2. The image will appear as far behind the mirror as the object’s distance is in front of ...
... 1. A light ray is an imaginary line that represents a thin beam of light in a ray diagram. Drawing light rays is helpful in predicting the location and size of an image that will be created by a mirror or lens. 2. The image will appear as far behind the mirror as the object’s distance is in front of ...
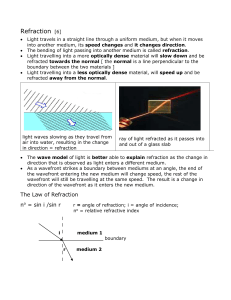
Refraction and the speed of light
... atomic electrons, traveling at speed c, shifts the phase of the radiation in the air downstream of the glass in the same way that would occur if the light were to go slower than c in the glass, with a shorter wavelength and an index of refraction greater than one for frequencies below the natural fr ...
... atomic electrons, traveling at speed c, shifts the phase of the radiation in the air downstream of the glass in the same way that would occur if the light were to go slower than c in the glass, with a shorter wavelength and an index of refraction greater than one for frequencies below the natural fr ...
Transparent and translucent casting resins for LED
... to protect these sensitive lighting elements against mechanical damage, moisture and other environmental influences. The specific requirements of the part determine the selection of the suitable potting system. The potting properties can be adapted to the specific application and part features, in p ...
... to protect these sensitive lighting elements against mechanical damage, moisture and other environmental influences. The specific requirements of the part determine the selection of the suitable potting system. The potting properties can be adapted to the specific application and part features, in p ...
PHYA2 INT DIFF_Q
... shown in the figure below. Shown are some line spectra for six elements that have been obtained in the laboratory. Place ticks in the boxes next to the three elements that are present in the atmosphere of star X. ...
... shown in the figure below. Shown are some line spectra for six elements that have been obtained in the laboratory. Place ticks in the boxes next to the three elements that are present in the atmosphere of star X. ...
Plants, Light, and LEDs
... Called kanban in Japan where it was developed, this is a method of inventory control that keeps inventories low by scheduling parts and supplies to arrive at the factory a short time before a production run begins. ...
... Called kanban in Japan where it was developed, this is a method of inventory control that keeps inventories low by scheduling parts and supplies to arrive at the factory a short time before a production run begins. ...
Oppgave 5.
... Predict the 13C-NMR chemical shift values for the following compounds. For compound a): Use tables and charts posted or displayed in the texbook AND Chemdraw. For compounds b-e): use Chemdraw only. a) b) c) d) e) ...
... Predict the 13C-NMR chemical shift values for the following compounds. For compound a): Use tables and charts posted or displayed in the texbook AND Chemdraw. For compounds b-e): use Chemdraw only. a) b) c) d) e) ...
Thomas Young and the Wav...ure of Light - OpenMind copia
... who established the principle of interference of light and thus resurrected the century-old wave theory of light. He was also an Egyptologist who helped decipher the Rosetta Stone.» In fact, Young held discoveries in virtually every field that he studied, including physics (the wave theory of light) ...
... who established the principle of interference of light and thus resurrected the century-old wave theory of light. He was also an Egyptologist who helped decipher the Rosetta Stone.» In fact, Young held discoveries in virtually every field that he studied, including physics (the wave theory of light) ...
Grade 10 Optics Unit Outline - RosedaleGrade10Science
... Images produced by Lenses: Drawing ray diagrams for converging lens ...
... Images produced by Lenses: Drawing ray diagrams for converging lens ...
specimen
... The rotating mirror method was much improved and developed over the next half century, culminating in two sets of measurements by Michelson and colleagues. These used rotating mirrors, but the mirrors were made 8-sided so that Michelson could look for the speed of rotation at which the light reflect ...
... The rotating mirror method was much improved and developed over the next half century, culminating in two sets of measurements by Michelson and colleagues. These used rotating mirrors, but the mirrors were made 8-sided so that Michelson could look for the speed of rotation at which the light reflect ...
light - Cloudfront.net
... • Ray = beam of electromagnetic radiation (light) • Normal = imaginary line that is perpendicular to the surface of an object • Incident Ray = emitted or incoming light ray • Reflected Ray = light ray that bounces off a surface; outgoing wave • Angle of Incidence = angle at which the incoming light ...
... • Ray = beam of electromagnetic radiation (light) • Normal = imaginary line that is perpendicular to the surface of an object • Incident Ray = emitted or incoming light ray • Reflected Ray = light ray that bounces off a surface; outgoing wave • Angle of Incidence = angle at which the incoming light ...
Photopolymer
A photopolymer is a polymer that changes its properties when exposed to light, often in the ultraviolet or visible region of the electromagnetic spectrum. These changes are often manifested structurally, for example hardening of the material occurs as a result of cross-linking when exposed to light. An example is shown below depicting a mixture of monomers, oligomers, and photoinitiators that conform into a hardened polymeric material through a process called curing,.A wide variety of technologically useful applications rely on photopolymers, for example some enamels and varnishes depend on photopolymer formulation for proper hardening upon exposure to light. In some instances, an enamel can cure in a fraction of a second when exposed to light, as opposed to thermally cured enamels which can require half an hour or longer. Curable materials are widely used for medical, printing, and photoresist technologies. Changes in structural and chemical properties can be induced internally by chromophores that the polymer subunit already possesses, or externally by addition of photosensitive molecules. Typically a photopolymer consists of a mixture of multifunctional monomers and oligomers in order to achieve the desired physical properties, and therefore a wide variety of monomers and oligomers have been developed that can polymerize in the presence of light either through internal or external initiation. Photopolymers undergo a process called curing, where oligomers are cross-linked upon exposure to light, forming what is known as a network polymer. The result of photo curing is the formation of a thermoset network of polymers. One of the advantages of photo-curing is that it can be done selectively using high energy light sources, for example lasers, however, most systems are not readily activated by light, and in this case a photoinitiator is required. Photoinitiators are compounds that upon radiation of light decompose into reactive species that activate polymerization of specific functional groups on the oligomers. An example of a mixture that undergoes cross-linking when exposed to light is shown below. The mixture consists of monomeric styrene and oligomeric acrylates.Most commonly, photopolymerized systems are typically cured through UV radiation, since ultraviolet light is more energetic; however, the development of dye-based photoinitiator systems have allowed for the use of visible light, having potential advantages of processes that are more simple and safe to handle. UV curing in industrial processes has greatly expanded over the past several decades. Many traditional thermally cured and solvent-based technologies can be replaced by photopolymerization technologies. The advantages of photopolymerization over thermally cured polymerization include high rates of polymerization and environmental benefits from elimination of volatile organic solvents.There are two general routes for photoinitiation: free radical and ionic. The general process involves doping a batch of neat polymer with small amounts of photoinitiator, followed by selective radiation of light, resulting a highly cross-linked product. Many of these reactions do not require solvent which eliminates termination path via reaction of initiators with solvent and impurities, in addition to decreasing the overall cost.