design synthesis and functionalization of self assembled
... architectures of different shapes and sizes, which can be modulated though judicious choice of metal and ligands. In addition to that the functionalization of supramolecular assemblies has also been extensively investigated over the past few years with an aim to develop nanoscale ensembles that can ...
... architectures of different shapes and sizes, which can be modulated though judicious choice of metal and ligands. In addition to that the functionalization of supramolecular assemblies has also been extensively investigated over the past few years with an aim to develop nanoscale ensembles that can ...
Chapter 1 Introduction to Forensic Chemistry
... The unit of a measurement contains critical information about what system of measurement is used and whether the base unit is modified with a prefix. ...
... The unit of a measurement contains critical information about what system of measurement is used and whether the base unit is modified with a prefix. ...
Complete Program - Mathematics and Computer Science
... result, students are provided with FIDs of necessary compounds. For example, during the unknown laboratory, each student is given both proton and carbon data for a solid and a liquid unknown. They use freeware to transform the data. As the second semester laboratory progresses, students are given FI ...
... result, students are provided with FIDs of necessary compounds. For example, during the unknown laboratory, each student is given both proton and carbon data for a solid and a liquid unknown. They use freeware to transform the data. As the second semester laboratory progresses, students are given FI ...
AQA Science GCSE Chemistry
... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners providing content and quality control, you can be confident that this course is as closely ...
... AQA recognizes the importance of good-quality teaching, learning and assessment resources to accompany their specification. That's why they've chosen to work exclusively with nelson Thornes. With AQA examiners providing content and quality control, you can be confident that this course is as closely ...
free sample
... 31) Which of the following pairs of aqueous solutions will form a precipitate when mixed? A) K2CO3 + NaCl B) Na2SO4 + KOH C) CaS + Na2SO4 D) None of these solution pairs will produce a precipitate. E) All of these solution pairs will produce a precipitate. Answer: C Diff: 1 ...
... 31) Which of the following pairs of aqueous solutions will form a precipitate when mixed? A) K2CO3 + NaCl B) Na2SO4 + KOH C) CaS + Na2SO4 D) None of these solution pairs will produce a precipitate. E) All of these solution pairs will produce a precipitate. Answer: C Diff: 1 ...
volume 2 - PianetaChimica
... tasks set in the IChO in its fourty-year history is a contribution of the IChO International Information Centre in Bratislava (Slovakia) to the development of this world known international competition. This Volume 2 contains 154 theoretical and 46 practical competition problems from the mentioned y ...
... tasks set in the IChO in its fourty-year history is a contribution of the IChO International Information Centre in Bratislava (Slovakia) to the development of this world known international competition. This Volume 2 contains 154 theoretical and 46 practical competition problems from the mentioned y ...
master ap chemistry - NelnetSolutions.com
... ALL RIGHTS RESERVED. No part of this work covered by the copyright herein may be reproduced or used in any form or by any means—graphic, electronic, or mechanical, including photocopying, recording, taping, Web distribution, or information storage and retrieval systems—without the prior written perm ...
... ALL RIGHTS RESERVED. No part of this work covered by the copyright herein may be reproduced or used in any form or by any means—graphic, electronic, or mechanical, including photocopying, recording, taping, Web distribution, or information storage and retrieval systems—without the prior written perm ...
analytical applications of surface-modified fused silica capillaries
... Fused silica capillaries have become a major tool for many applications in analytical chemistry. These capillaries are physically robust, permit gasses or solutions to be introduced and pumped through with relative ease, and due to their small dimensions allow fast mass transfer to and from the capi ...
... Fused silica capillaries have become a major tool for many applications in analytical chemistry. These capillaries are physically robust, permit gasses or solutions to be introduced and pumped through with relative ease, and due to their small dimensions allow fast mass transfer to and from the capi ...
volume 2 - HotNews
... tasks set in the ICHO in its fourty-year history is a contribution of the ICHO International Information Centre in Bratislava (Slovakia) to the development of this world known international competition. This Volume 2 contains 154 theoretical and 46 practical competition problems from the mentioned y ...
... tasks set in the ICHO in its fourty-year history is a contribution of the ICHO International Information Centre in Bratislava (Slovakia) to the development of this world known international competition. This Volume 2 contains 154 theoretical and 46 practical competition problems from the mentioned y ...
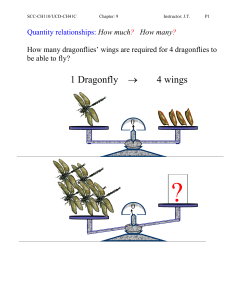
Quantity relationships: How much
... A mixture of 5.0 g of H2 (g) and 10.0 g of O2(g) is ignited. Water forms according to the following combination reaction: 2H2(g) +O2(g) → 2H2O(g) Which reactant is limiting? How much water will the reaction produce? ...
... A mixture of 5.0 g of H2 (g) and 10.0 g of O2(g) is ignited. Water forms according to the following combination reaction: 2H2(g) +O2(g) → 2H2O(g) Which reactant is limiting? How much water will the reaction produce? ...
Moles 1 - pedagogics.ca
... and stoichiometry is the study of the ratios in which chemical substances combine. In order to know the exact quantity of each substance that is required to react we need to know the number of atoms, molecules or ions present in a specific amount of that substance. However, the mass of an individual ...
... and stoichiometry is the study of the ratios in which chemical substances combine. In order to know the exact quantity of each substance that is required to react we need to know the number of atoms, molecules or ions present in a specific amount of that substance. However, the mass of an individual ...
Chapter+12
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
Stoichiometric Calculations
... The limiting reactant, or limiting reagent, is the reactant present in the smallest stoichiometric amount. This is not necessarily the one with the smallest mass. The limiting reactant is the reactant you’ll run out of first, and it is the one that determines the maximum amount of product that can b ...
... The limiting reactant, or limiting reagent, is the reactant present in the smallest stoichiometric amount. This is not necessarily the one with the smallest mass. The limiting reactant is the reactant you’ll run out of first, and it is the one that determines the maximum amount of product that can b ...
IB Chemistry Online SAQ_Ans
... c From the equation, amount of H2SO4 = amount of NaOH ÷ 2 = 0.0125 mol in 20.0 cm3, so ‘scaling up’ to 1000 cm3 to obtain the concentration of diluted sulfuric acid 1000 × 0.0125mol dm −3 ...
... c From the equation, amount of H2SO4 = amount of NaOH ÷ 2 = 0.0125 mol in 20.0 cm3, so ‘scaling up’ to 1000 cm3 to obtain the concentration of diluted sulfuric acid 1000 × 0.0125mol dm −3 ...
CBSE (Mains)
... (3) Smaller female is easily recognisable from larger male (4) It completes life cycle in about two weeks Sol: Ans [4] 52. The lac operon consists of : (1) Four regulatory genes only (2) One regulatory gene and three structural genes (3) Two regulatory genes and two structural genes (4) Three regula ...
... (3) Smaller female is easily recognisable from larger male (4) It completes life cycle in about two weeks Sol: Ans [4] 52. The lac operon consists of : (1) Four regulatory genes only (2) One regulatory gene and three structural genes (3) Two regulatory genes and two structural genes (4) Three regula ...
mcdonald (pam78654) – HW 1: High School Concepts – laude
... mcdonald (pam78654) – HW 1: High School Concepts – laude – (89560) This print-out should have 40 questions. Multiple-choice questions may continue on the next column or page – find all choices before answering. 001 10.0 points Calculate the number of H2 O molecules in 1.00 cm3 of water at 0◦ C (dens ...
... mcdonald (pam78654) – HW 1: High School Concepts – laude – (89560) This print-out should have 40 questions. Multiple-choice questions may continue on the next column or page – find all choices before answering. 001 10.0 points Calculate the number of H2 O molecules in 1.00 cm3 of water at 0◦ C (dens ...












![1 Solutions 4a (Chapter 4 problems) Chem151 [Kua]](http://s1.studyres.com/store/data/002731518_1-574ec10e88e667508364281b6325aeef-300x300.png)