Teacher Edition Calculations
... terms of mole ratios Compare mass changes in samples of metals when they combine with oxygen Perform a first-hand investigation to meas ure and identify the mass ratios of metal to non metal(s) in a common compound and calculate its empirical formula Describe the contribution of Gay-Lussac to the un ...
... terms of mole ratios Compare mass changes in samples of metals when they combine with oxygen Perform a first-hand investigation to meas ure and identify the mass ratios of metal to non metal(s) in a common compound and calculate its empirical formula Describe the contribution of Gay-Lussac to the un ...
Stoichiometry of Formulas and Equations
... the amounts of substances consumed and produced in a reaction. Suppose you are a polymer chemist preparing a new plastic: how much of this new material will a given polymerization reaction yield? Or suppose you’re a chemical engineer studying rocket engine thrust: what amount of exhaust gases will a ...
... the amounts of substances consumed and produced in a reaction. Suppose you are a polymer chemist preparing a new plastic: how much of this new material will a given polymerization reaction yield? Or suppose you’re a chemical engineer studying rocket engine thrust: what amount of exhaust gases will a ...
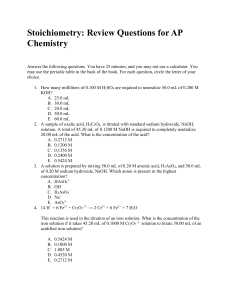
SCH3U: Final Exam Review Note: These questions a
... Note: These questions are just to help you prepare for the exam. This review should be the minimum that you do to prepare for the exam. The solutions to the review questions are at the back of the handout. UNIT: Matter and Chemical Bonding A) Elements and the Periodic Table 1. How many protons, neut ...
... Note: These questions are just to help you prepare for the exam. This review should be the minimum that you do to prepare for the exam. The solutions to the review questions are at the back of the handout. UNIT: Matter and Chemical Bonding A) Elements and the Periodic Table 1. How many protons, neut ...
Solutions - ChemConnections
... ∆GE = !RT ln K = ∆HE ! T∆SE; HX(aq) ⇌ H+(aq) + X−(aq) Ka reaction; the value of Ka for HF is less than one, while the other hydrogen halide acids have Ka > 1. In terms of ∆GE, HF must have a positive ∆G orxn value, while the other HX acids have ∆G°rxn < 0. The reason for the sign change in the Ka va ...
... ∆GE = !RT ln K = ∆HE ! T∆SE; HX(aq) ⇌ H+(aq) + X−(aq) Ka reaction; the value of Ka for HF is less than one, while the other hydrogen halide acids have Ka > 1. In terms of ∆GE, HF must have a positive ∆G orxn value, while the other HX acids have ∆G°rxn < 0. The reason for the sign change in the Ka va ...
Chapter 1
... appear in only one reactant and product, by adjusting stoichiometric coefficients. Generally, H and O are balanced last. When balancing an equation, start by determining the number of atoms on each side of the chemical equation: (a) Start by determining the chemical formula of each compound. Molecul ...
... appear in only one reactant and product, by adjusting stoichiometric coefficients. Generally, H and O are balanced last. When balancing an equation, start by determining the number of atoms on each side of the chemical equation: (a) Start by determining the chemical formula of each compound. Molecul ...
Chapter 15 Calculations in chemistry: stoichiometry
... Lead(II) chromate has been used as a bright yellow pigment in some paints. It can be produced by the reaction of potassium chromate with lead nitrate. a Write a full equation for this reaction. b What mass of potassium chromate is required to produce 6.0 g of lead chromate? c Suggest a reason why le ...
... Lead(II) chromate has been used as a bright yellow pigment in some paints. It can be produced by the reaction of potassium chromate with lead nitrate. a Write a full equation for this reaction. b What mass of potassium chromate is required to produce 6.0 g of lead chromate? c Suggest a reason why le ...
Soln Chem 2008Nov(9746)
... When the pack is squeezed, NH4NO3(s) dissolves in the water suggests that the reaction is spontaneous; i.e. ∆G is negative. Dissolution of NH4NO3(s) is accompanied by an increase in entropy (less orderly); i.e. ∆S is positive. Hence, option C. (ans) © Step-by-Step ...
... When the pack is squeezed, NH4NO3(s) dissolves in the water suggests that the reaction is spontaneous; i.e. ∆G is negative. Dissolution of NH4NO3(s) is accompanied by an increase in entropy (less orderly); i.e. ∆S is positive. Hence, option C. (ans) © Step-by-Step ...
b - Gordon State College
... Procedure for limiting/excess reagent calculations aA + bB cC + dD 1) Make sure the equation is balanced. 2) Find the moles of each reactant: moles = mass in gram / molar mass 3) Pick up any reactant, say A, and use the stoichiometry to calculate the required amount of the other reactant B. 4) Co ...
... Procedure for limiting/excess reagent calculations aA + bB cC + dD 1) Make sure the equation is balanced. 2) Find the moles of each reactant: moles = mass in gram / molar mass 3) Pick up any reactant, say A, and use the stoichiometry to calculate the required amount of the other reactant B. 4) Co ...
SCH3U: Final Exam Review
... ____ iii. When the volume of a gas is decreased by a factor of 5, the temperature is decreased by a factor of 5. ____ iv. When the pressure of a gas is halved, the temperature is halved. ____ v. When the volume of a gas is increased by a factor of 5,the temperature is decreased by a factor of 5. B) ...
... ____ iii. When the volume of a gas is decreased by a factor of 5, the temperature is decreased by a factor of 5. ____ iv. When the pressure of a gas is halved, the temperature is halved. ____ v. When the volume of a gas is increased by a factor of 5,the temperature is decreased by a factor of 5. B) ...
for the exam on 14 feb
... To reach the next equivalence point, you’d need another equivalence of NaOH (in other words, to reach the first equivalence point, you needed 0.00100 mol, and to reach the second equivalence point, you’d need 0.00200 mol – double the first.) You’re only halfway to the second equivalence point, here ...
... To reach the next equivalence point, you’d need another equivalence of NaOH (in other words, to reach the first equivalence point, you needed 0.00100 mol, and to reach the second equivalence point, you’d need 0.00200 mol – double the first.) You’re only halfway to the second equivalence point, here ...
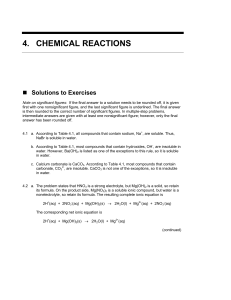
4. chemical reactions
... Some electrolyte solutions are strongly conducting because they are almost completely ionized and others are weakly conducting because they are weakly ionized. The former solutions will have many more ions to conduct electricity than will the latter solutions if both are present at the same concentr ...
... Some electrolyte solutions are strongly conducting because they are almost completely ionized and others are weakly conducting because they are weakly ionized. The former solutions will have many more ions to conduct electricity than will the latter solutions if both are present at the same concentr ...
Chapter 12
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
... Makes 3 dozen If we had the specified amount of all ingredients listed, could we make 4 dozen cookies? What if we had 6 eggs and twice as much of everything else, could we make 9 dozen cookies? What if we only had one egg, could we make 3 dozen cookies? ...
SCH4U TEXT BOOK
... foods that have been grown without the use of pesticides, herbicides, fertilizers, hormones, and other synthetic chemicals. The original meaning of the word “organic” refers to anything that is or has been alive. In this sense, all vegetables are organic, no matter how they are grown. Organic chemis ...
... foods that have been grown without the use of pesticides, herbicides, fertilizers, hormones, and other synthetic chemicals. The original meaning of the word “organic” refers to anything that is or has been alive. In this sense, all vegetables are organic, no matter how they are grown. Organic chemis ...
CHAPTER 9
... A balanced chemical equation is the key step in all stoichiometric calculations, because the mole ratio is obtained directly from it. Solving any reaction stoichiometry problem must begin with a balanced equation. Chemical equations help us plan the amounts of reactants to use in a chemical reaction ...
... A balanced chemical equation is the key step in all stoichiometric calculations, because the mole ratio is obtained directly from it. Solving any reaction stoichiometry problem must begin with a balanced equation. Chemical equations help us plan the amounts of reactants to use in a chemical reaction ...
CHAPTER 5 GASES
... Recall that Avogadro’s Law states that the volume of a gas is directly proportional to the number of moles of gas at constant temperature and pressure. The ammonia and nitric oxide coefficients in the balanced equation are the same, so one volume of nitric oxide must be obtained from one volume of a ...
... Recall that Avogadro’s Law states that the volume of a gas is directly proportional to the number of moles of gas at constant temperature and pressure. The ammonia and nitric oxide coefficients in the balanced equation are the same, so one volume of nitric oxide must be obtained from one volume of a ...
Brief Contents - Educhimica.it
... and the second number stops its significant figure in the hundredths place after the decimal. Hence, we limit our final answer to the tenths place after the decimal. The final answer is 59.4. b. 0.00665 + 1.004 = 1.01065. The first number stops its significant figure in the ten thousandths place after the ...
... and the second number stops its significant figure in the hundredths place after the decimal. Hence, we limit our final answer to the tenths place after the decimal. The final answer is 59.4. b. 0.00665 + 1.004 = 1.01065. The first number stops its significant figure in the ten thousandths place after the ...