Ch9Overview9-1KEY
... is exergonic, releasing 686 kcal/mol of glucose decomposed is a redox reaction: glucose is oxidized, while oxygen is reduced transfers electrons to a lower energy state, liberating energy consists of many steps, each one catalyzed by an enzyme, so that the energy released is harnessed efficiently is ...
... is exergonic, releasing 686 kcal/mol of glucose decomposed is a redox reaction: glucose is oxidized, while oxygen is reduced transfers electrons to a lower energy state, liberating energy consists of many steps, each one catalyzed by an enzyme, so that the energy released is harnessed efficiently is ...
AP Biology Cell Respiration Quiz Study Guide
... 5. What is the final electron acceptor in the electron transport chain? 6. From what macromolecules would you obtain the highest amount of ATP? 7. What is chemiosmosis? 8. Which respiratory process generates the most ATP? 9. Why is ATP such a useful energy storage/transfer molecule? 10. How is the e ...
... 5. What is the final electron acceptor in the electron transport chain? 6. From what macromolecules would you obtain the highest amount of ATP? 7. What is chemiosmosis? 8. Which respiratory process generates the most ATP? 9. Why is ATP such a useful energy storage/transfer molecule? 10. How is the e ...
CHM 365 Name: Exam 3 Do all of the following 21 questions
... e) absolute requirement for ATP hydrolysis, no other energy source will work. ...
... e) absolute requirement for ATP hydrolysis, no other energy source will work. ...
SI Practice Exam / Review Sheet
... 4. The concentration of ligand at which fifty percent of receptors are bound is known as the _____________________. 5. A protein that becomes functional after undergoing a conformational change is a/an ________________ protein. 6. Protein Kinase A is activated by ______________. 7. In metabolism, sy ...
... 4. The concentration of ligand at which fifty percent of receptors are bound is known as the _____________________. 5. A protein that becomes functional after undergoing a conformational change is a/an ________________ protein. 6. Protein Kinase A is activated by ______________. 7. In metabolism, sy ...
AP Biology Question Set
... 48. The conversion of ATP to ADP and Pi releases approxi mately 7.3 kcal/mol of energy. This energy release fuels (endergonic) reactions in the cell. Equilibrium of the reaction is far to the right and favors the formation of ADP. In the converse, the formation of ATP from ADP and Pi is energy inten ...
... 48. The conversion of ATP to ADP and Pi releases approxi mately 7.3 kcal/mol of energy. This energy release fuels (endergonic) reactions in the cell. Equilibrium of the reaction is far to the right and favors the formation of ADP. In the converse, the formation of ATP from ADP and Pi is energy inten ...
role of respiration in glycolysis, co2 and h20 production
... Set of the metabolic reactions that occur in cells to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions that involve the oxidation of one molecule and the reduction of another. ...
... Set of the metabolic reactions that occur in cells to convert biochemical energy from nutrients into adenosine triphosphate (ATP), and then release waste products. The reactions involved in respiration are catabolic reactions that involve the oxidation of one molecule and the reduction of another. ...
1 Chapter 5 Microbial Metabolism 2
... Glucose + 2 ATP + 2 ADP + 2 PO4– + 2 NAD+ → 2 pyruvic acid + 4 ATP + 2 NADH + 2H+ Alternatives to Glycolysis Pentose phosphate pathway Uses pentoses and NADPH Operates with glycolysis Entner-Doudoroff pathway Produces NADPH and ATP Does not involve glycolysis Pseudomonas, Rhizobium, Agrobacterium Cel ...
... Glucose + 2 ATP + 2 ADP + 2 PO4– + 2 NAD+ → 2 pyruvic acid + 4 ATP + 2 NADH + 2H+ Alternatives to Glycolysis Pentose phosphate pathway Uses pentoses and NADPH Operates with glycolysis Entner-Doudoroff pathway Produces NADPH and ATP Does not involve glycolysis Pseudomonas, Rhizobium, Agrobacterium Cel ...
presentation source
... Fate of pyruvic acid in muscles • Pyruvic acid is reduced by NADH forming a molecule of lactic acid. • C3H4O3 + NADH + H+ -> C3H6O3 + NAD+ • The process is called lactic acid fermentation. • The process is energetically wasteful because so much free energy remains in the lactic acid molecule. (It c ...
... Fate of pyruvic acid in muscles • Pyruvic acid is reduced by NADH forming a molecule of lactic acid. • C3H4O3 + NADH + H+ -> C3H6O3 + NAD+ • The process is called lactic acid fermentation. • The process is energetically wasteful because so much free energy remains in the lactic acid molecule. (It c ...
ADP, ATP and Cellular Respiration Powerpoint
... mitochondria will undergo aerobic respiration which leads to the Krebs cycle. However, if oxygen is not present, fermentation of the pyruvate molecule will occur. In the presence of oxygen, when acetyl-CoA is produced, the molecule then enters the citric acid cycle (Krebs cycle) ...
... mitochondria will undergo aerobic respiration which leads to the Krebs cycle. However, if oxygen is not present, fermentation of the pyruvate molecule will occur. In the presence of oxygen, when acetyl-CoA is produced, the molecule then enters the citric acid cycle (Krebs cycle) ...
Molecular Structure and Physiological Function of Chloride
... Extreme outward rectification Inhibition by extracellular acidic pH ...
... Extreme outward rectification Inhibition by extracellular acidic pH ...
Clostridia
... some of the carboxylic acid groups are esterified with methanol. During fermentation, the ester groups are hydrolyzed, and methanol is released ...
... some of the carboxylic acid groups are esterified with methanol. During fermentation, the ester groups are hydrolyzed, and methanol is released ...
BCOR 011 Exam 2, 2004
... 8. How does an enzyme catalyze a reaction? A. by changing the equilibrium of a spontaneous reaction B. by lowering the energy of activation of a reaction C. by supplying the energy to speed up a reaction D. by lowering the ∆G of a reaction E. by increasing the amount of free energy of a reaction 9. ...
... 8. How does an enzyme catalyze a reaction? A. by changing the equilibrium of a spontaneous reaction B. by lowering the energy of activation of a reaction C. by supplying the energy to speed up a reaction D. by lowering the ∆G of a reaction E. by increasing the amount of free energy of a reaction 9. ...
Chapter 9 Notes: Cellular Respiration
... iii. This process is anaerobic – it does not require oxygen b. Steps of Oxidative Respiration: i. This process is aerobic- it requires oxygen ii. Pyruvate is broken down into pyruvic acid. iii. Krebs Cycle - pyruvic acid is broken down into CO2 in a series of energy-extracting reactions; high-energy ...
... iii. This process is anaerobic – it does not require oxygen b. Steps of Oxidative Respiration: i. This process is aerobic- it requires oxygen ii. Pyruvate is broken down into pyruvic acid. iii. Krebs Cycle - pyruvic acid is broken down into CO2 in a series of energy-extracting reactions; high-energy ...
Lecture 7-enzymes 3
... Oxygenases Oxygenases catalyze substrate oxidation by molecular O2 The reduced product of the reaction in this case is water and not hydrogen peroxide There are two types of oxygenases: Monooxygenases; transfer one oxygen atom to the substrate, and reduce the other oxygen atom to water Di ...
... Oxygenases Oxygenases catalyze substrate oxidation by molecular O2 The reduced product of the reaction in this case is water and not hydrogen peroxide There are two types of oxygenases: Monooxygenases; transfer one oxygen atom to the substrate, and reduce the other oxygen atom to water Di ...
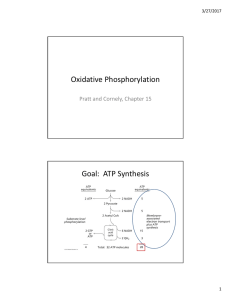
Oxidative Phosphorylation Goal: ATP Synthesis
... half reactions below to calculate the standard free energy change. How can you account for the fact that this process is spontaneous in the cell? ...
... half reactions below to calculate the standard free energy change. How can you account for the fact that this process is spontaneous in the cell? ...
Full_ppt_ch23
... Reaction 6: Dehyrogenation • In this oxidation, two H are removed from succinate to form a double bond in fumarate • FAD is reduced to FADH2 ...
... Reaction 6: Dehyrogenation • In this oxidation, two H are removed from succinate to form a double bond in fumarate • FAD is reduced to FADH2 ...
ADP, ATP and Cellular Respiration Powerpoint
... The pyruvic acid formed during glycolysis is broken down to lactic acid and energy is released (which is used to form ATP). Glucose → Pyruvic acid → Lactic acid + energy ...
... The pyruvic acid formed during glycolysis is broken down to lactic acid and energy is released (which is used to form ATP). Glucose → Pyruvic acid → Lactic acid + energy ...
MULTIPLE CHOICE. Choose the one alternative that best
... D. His cells lack the enzyme in glycolysis that forms pyruvate. E. His cells cannot move NADH from glycolysis into the mitochondria. 18. In chemiosmotic phosphorylation, what is the most direct source of energy that is used to convert ADP + Pi to ATP? A. energy released as electrons flow through the ...
... D. His cells lack the enzyme in glycolysis that forms pyruvate. E. His cells cannot move NADH from glycolysis into the mitochondria. 18. In chemiosmotic phosphorylation, what is the most direct source of energy that is used to convert ADP + Pi to ATP? A. energy released as electrons flow through the ...
2421_Ch5.ppt
... Oxidation-Reduction reactions: reaction where one substrate loses electrons (oxidation) and the other gains electrons (reduction) ...
... Oxidation-Reduction reactions: reaction where one substrate loses electrons (oxidation) and the other gains electrons (reduction) ...
O 2 - Madison Public Schools
... proteins all catabolized through same pathways enter at different points cell extracts energy from every source ...
... proteins all catabolized through same pathways enter at different points cell extracts energy from every source ...
Ions - RCSD
... • Made of C, H, O, N • Held together by hydrogen bonding (peptide bonds) so heat, pH change, radiation, etc. affect the bonding ...
... • Made of C, H, O, N • Held together by hydrogen bonding (peptide bonds) so heat, pH change, radiation, etc. affect the bonding ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.