TABLE 3–1 Some Common Types of Enzymes
... catalyze the rearrangement of bonds within a single molecule. catalyze polymerization reactions such as the synthesis of DNA and RNA. catalyze the addition of phosphate groups to molecules. Protein kinases are an important group of kinases that attach phosphate groups to proteins. catalyze the hydro ...
... catalyze the rearrangement of bonds within a single molecule. catalyze polymerization reactions such as the synthesis of DNA and RNA. catalyze the addition of phosphate groups to molecules. Protein kinases are an important group of kinases that attach phosphate groups to proteins. catalyze the hydro ...
Bio102 Problems
... lipid must be more oxidized more times than each carbon atom from a carbohydrate. With each of these oxidations, a reduced coenzyme is produced which will ultimately be used to synthesize more ATP by oxidative phosphorylation. 16. The AMPK enzyme becomes active when A. PFK activity is inhibited. B. ...
... lipid must be more oxidized more times than each carbon atom from a carbohydrate. With each of these oxidations, a reduced coenzyme is produced which will ultimately be used to synthesize more ATP by oxidative phosphorylation. 16. The AMPK enzyme becomes active when A. PFK activity is inhibited. B. ...
HOW CELLS HARVEST ENERGY
... High ATP means cell has enuf ATP and ATP acts like allosteric inhibitor to turn off 2nd enzyme in glycolysis (phosphofructokinase) High NADH means too much glucose is being broken down and the cell has enuf ATP it acts as allosteric inhibitor and inhibits oxidation of pyruvate High ADP means cell ne ...
... High ATP means cell has enuf ATP and ATP acts like allosteric inhibitor to turn off 2nd enzyme in glycolysis (phosphofructokinase) High NADH means too much glucose is being broken down and the cell has enuf ATP it acts as allosteric inhibitor and inhibits oxidation of pyruvate High ADP means cell ne ...
Electron Carriers
... The energy released by electrons moving down the chain is used to pump H+ from the matrix to the intermembrane space This creates a proton gradient (potential energy) ...
... The energy released by electrons moving down the chain is used to pump H+ from the matrix to the intermembrane space This creates a proton gradient (potential energy) ...
Final Review - Chemistry Courses: About: Department of
... 15. purpose of light reactions, dark reactions 16. energetics of FA synthesis and degradation 17. nitrogen processing, catabolism of AA 18. medical applications of nucleotide metabolism 19. nucleic acid structure on atomic level ...
... 15. purpose of light reactions, dark reactions 16. energetics of FA synthesis and degradation 17. nitrogen processing, catabolism of AA 18. medical applications of nucleotide metabolism 19. nucleic acid structure on atomic level ...
Chapter 5: Microbial Metabolism
... molecules so the enzyme can "find" its substrate. Lower temperatures will decrease the rate of collisions and the rate of reactions. Increased temperatures will denature the enzyme. 16. Ethyl alcohol, lactic acid, butyl alcohol, acetone, and glycerol are some of the possible products. Refer to Table ...
... molecules so the enzyme can "find" its substrate. Lower temperatures will decrease the rate of collisions and the rate of reactions. Increased temperatures will denature the enzyme. 16. Ethyl alcohol, lactic acid, butyl alcohol, acetone, and glycerol are some of the possible products. Refer to Table ...
Sunday School Jeopardy - Chapman @ Norquay School
... name of the process used to do this? The high-energy phosphate bond (the third one) is broken by hydrolysis. ...
... name of the process used to do this? The high-energy phosphate bond (the third one) is broken by hydrolysis. ...
Glycolysis, Krebs Cycle, and other Energy
... 1- Plants make ATP during photosynthesis. 2- All other organisms, including plants, must produce ATP by breaking down molecules such as glucose. Aerobic respiration : the process by which a cell uses O2 to "burn" molecules and release energy. C6H12O6 + 6O2 6CO2 + 6H2O Note: this reaction is the o ...
... 1- Plants make ATP during photosynthesis. 2- All other organisms, including plants, must produce ATP by breaking down molecules such as glucose. Aerobic respiration : the process by which a cell uses O2 to "burn" molecules and release energy. C6H12O6 + 6O2 6CO2 + 6H2O Note: this reaction is the o ...
Glycolysis - Fairfield Public Schools
... strip off H+ ions to be used in oxidative phosphorylation. A total of 2 ATP molecules are formed by substrate level phosphorylation. The cycle oxidizes pyruvate to 6 NADH, and 2 FADH2 The remaining carbon and oxygen are released as CO2 (4 CO2 total). ...
... strip off H+ ions to be used in oxidative phosphorylation. A total of 2 ATP molecules are formed by substrate level phosphorylation. The cycle oxidizes pyruvate to 6 NADH, and 2 FADH2 The remaining carbon and oxygen are released as CO2 (4 CO2 total). ...
acetyl-CoA - Winona State University
... positive value if there is “No Membrane In Between”. This is why Delta G from the reactions in the mitochondria cannot help to drive the reactions of glycolysis in the cytosol. Although molecules such as pyruvate can “carry” the energy between different compartments. ...
... positive value if there is “No Membrane In Between”. This is why Delta G from the reactions in the mitochondria cannot help to drive the reactions of glycolysis in the cytosol. Although molecules such as pyruvate can “carry” the energy between different compartments. ...
Problem Set #3 Key
... inhibitor of an enzyme associated with metabolism. After eating these delectable brownies, Chris finds that only moles of 48 ATP are being produced per mole of sucrose. Which enzyme does the inhibitor act upon? Be sure to name the inhibited enzyme and the pathway with which it is usually associated. ...
... inhibitor of an enzyme associated with metabolism. After eating these delectable brownies, Chris finds that only moles of 48 ATP are being produced per mole of sucrose. Which enzyme does the inhibitor act upon? Be sure to name the inhibited enzyme and the pathway with which it is usually associated. ...
9.2 Krebs Cycle and Electron Transport Reading Guide
... During electron transport, H+ ions build up in the intermembrane space, making it positively charged. The other side of the membrane, from which those H+ ions have been taken, is now negatively charged. The charge differences that build up cause the ions to move. the matrix? ...
... During electron transport, H+ ions build up in the intermembrane space, making it positively charged. The other side of the membrane, from which those H+ ions have been taken, is now negatively charged. The charge differences that build up cause the ions to move. the matrix? ...
bme-biochem-5-1-atp-adp-cycle-kh-6
... ATP PRODUCTION FROM CARBOHYDRATES Electron Transport Chain A series of Oxidative Phosphorylation reactions Oxidation = the removal of electrons from a molecule and results in a decrease in the energy content of the molecule. Because most biological reactions involve the loss of hydrogen atoms, they ...
... ATP PRODUCTION FROM CARBOHYDRATES Electron Transport Chain A series of Oxidative Phosphorylation reactions Oxidation = the removal of electrons from a molecule and results in a decrease in the energy content of the molecule. Because most biological reactions involve the loss of hydrogen atoms, they ...
File - Ms. Daley Science
... for some tests. There they discover his mitochondria can use only fatty acids and amino acids for respiration, and his cells produce more lactic acid than normal. Of the following, which is the best explanation of his condition? (a)) His mitochondria lack the transport protein that moves pyruvate ac ...
... for some tests. There they discover his mitochondria can use only fatty acids and amino acids for respiration, and his cells produce more lactic acid than normal. Of the following, which is the best explanation of his condition? (a)) His mitochondria lack the transport protein that moves pyruvate ac ...
Chapter 9: Cellular Respiration and Fermentation - Biology E
... The ATP synthase harnesses the proton-motive force to phosphorylate ADP, forming ATP. Together, electron transport and chemiosmosis make up oxidative phosphorylation. 31. To account for the total number of ATPs that could be formed from a glucose molecule, we have to add the substrate-level ATPs fr ...
... The ATP synthase harnesses the proton-motive force to phosphorylate ADP, forming ATP. Together, electron transport and chemiosmosis make up oxidative phosphorylation. 31. To account for the total number of ATPs that could be formed from a glucose molecule, we have to add the substrate-level ATPs fr ...
Cellular Respiration
... Sometimes energy is required to transport NADH + H+ formed by glycolysis from the cytoplasm through the inner mitochondrial membrane. Some H+ in chemiosmosis may leak through the membrane. • Aerobic Respiration is generally 19 times more efficient than anaerobic respiration. • The ATP produced d ...
... Sometimes energy is required to transport NADH + H+ formed by glycolysis from the cytoplasm through the inner mitochondrial membrane. Some H+ in chemiosmosis may leak through the membrane. • Aerobic Respiration is generally 19 times more efficient than anaerobic respiration. • The ATP produced d ...
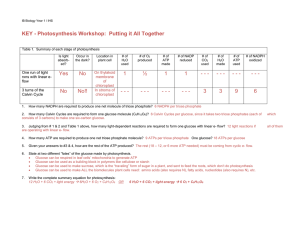
KEY - Photosynthesis Workshop: Putting it All Together
... consists of 3 carbons) to make one six-carbon glucose. ...
... consists of 3 carbons) to make one six-carbon glucose. ...
Chapter 10- Photosynthesis
... - Hydrogen ions from photolysis of water accumulate inside the thylakoid compartment of chloroplasts to set up concentration and electric gradients. - As the hydrogen ions flow out through channels into the stroma, enzyme action links phosphate to ADP to form ATP. 2. Light-Independent Reactions (Car ...
... - Hydrogen ions from photolysis of water accumulate inside the thylakoid compartment of chloroplasts to set up concentration and electric gradients. - As the hydrogen ions flow out through channels into the stroma, enzyme action links phosphate to ADP to form ATP. 2. Light-Independent Reactions (Car ...
Section 9.2 Summary – pages 225-230
... form ATP from ADP, or to pump hydrogen ions into the center of the thylakoid disc. • Electrons are re-energized in a second photosystem and passed down a second electron transport __________. ...
... form ATP from ADP, or to pump hydrogen ions into the center of the thylakoid disc. • Electrons are re-energized in a second photosystem and passed down a second electron transport __________. ...
Living organisms need a constant input of energy
... Glycolysis, Krebs, and the lot of it The metabolism of carbohydrate molecules in cells, particulary glucose, provides energy in the form of ATP. Through the glycolytic pathway, glucose is first converted to pyruvate, anaerobically, in the cytosol. In the absence of sufficient oxygen, in the cytosol, ...
... Glycolysis, Krebs, and the lot of it The metabolism of carbohydrate molecules in cells, particulary glucose, provides energy in the form of ATP. Through the glycolytic pathway, glucose is first converted to pyruvate, anaerobically, in the cytosol. In the absence of sufficient oxygen, in the cytosol, ...
Chapter 10- Photosynthesis
... - Hydrogen ions from photolysis of water accumulate inside the thylakoid compartment of chloroplasts to set up concentration and electric gradients. - As the hydrogen ions flow out through channels into the stroma, enzyme action links phosphate to ADP to form ATP. 2. Light-Independent Reactions (Car ...
... - Hydrogen ions from photolysis of water accumulate inside the thylakoid compartment of chloroplasts to set up concentration and electric gradients. - As the hydrogen ions flow out through channels into the stroma, enzyme action links phosphate to ADP to form ATP. 2. Light-Independent Reactions (Car ...
Lecture 023--Photosynthesis 2 (Dark Reactions)
... Calvin Cycle PGAL end product of Calvin energy rich sugar 3 carbon compound “C3 photosynthesis” ...
... Calvin Cycle PGAL end product of Calvin energy rich sugar 3 carbon compound “C3 photosynthesis” ...
2014 Cellular Respiration ppt
... Stages of Cellular Respiration 1. GLYCOLYSIS (fist step in both types of respiration) -- Enzymeassisted anaerobic process that breaks down one sixcarbon molecule of glucose to two three-carbon pyruvate ions, producing a net result of 2 ATP and a NADH, electron ...
... Stages of Cellular Respiration 1. GLYCOLYSIS (fist step in both types of respiration) -- Enzymeassisted anaerobic process that breaks down one sixcarbon molecule of glucose to two three-carbon pyruvate ions, producing a net result of 2 ATP and a NADH, electron ...
Cellular Respiration and Fermentation
... b) They evolved before photosynthesis and used electron acceptors other than oxygen. c) Individual enzymes were present before photosynthesis but served other functions, such as amino acid metabolism. d) They evolved when the ancestral eukaryotes acquired mitochondria. ...
... b) They evolved before photosynthesis and used electron acceptors other than oxygen. c) Individual enzymes were present before photosynthesis but served other functions, such as amino acid metabolism. d) They evolved when the ancestral eukaryotes acquired mitochondria. ...
Notes Chapter 7 Cellular Respiration
... energy and make ATP. It includes anaerobic pathways, which operate in the absence of oxygen, and aerobic respiration, which occurs when oxygen is present. Cellular respiration begins with glycolysis, which takes place in the cytosol of cells. During glycolysis, one glucose molecule is oxidized to ...
... energy and make ATP. It includes anaerobic pathways, which operate in the absence of oxygen, and aerobic respiration, which occurs when oxygen is present. Cellular respiration begins with glycolysis, which takes place in the cytosol of cells. During glycolysis, one glucose molecule is oxidized to ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.