chap18 oxidative phosphorylation
... aerobic conditions. Oxidative pphosphorylation produces 30 of the 32 molecules of ATP that are formed when glucose is oxidized to CO2 and H2O. The process is conceptually easy but mechanistically very difficult. The electron flow from NADH and FADH2 to oxygen through protein complexes leads to pumpi ...
... aerobic conditions. Oxidative pphosphorylation produces 30 of the 32 molecules of ATP that are formed when glucose is oxidized to CO2 and H2O. The process is conceptually easy but mechanistically very difficult. The electron flow from NADH and FADH2 to oxygen through protein complexes leads to pumpi ...
Oxidation – a molecule loses electrons
... a. All of the NADH and FADH2 molecules created in glycolysis and the Citric Acid Cycle become oxidized (lose their e-, therefore recycled back to NAD+ and FAD) to the proteins in the inner membrane of the mitochondria. While the electrons are passed from protein to protein, energy is released that i ...
... a. All of the NADH and FADH2 molecules created in glycolysis and the Citric Acid Cycle become oxidized (lose their e-, therefore recycled back to NAD+ and FAD) to the proteins in the inner membrane of the mitochondria. While the electrons are passed from protein to protein, energy is released that i ...
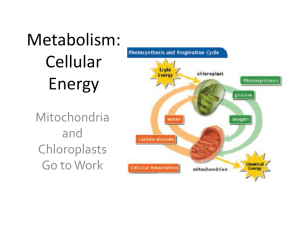
Cellular Energy
... • The life processes of all organisms require energy. • The potential energy held in the bonds of food molecules CANNOT be used directly by the cell. • Energy from food must be converted to the ONLY energy source that cells can use: ATP! ...
... • The life processes of all organisms require energy. • The potential energy held in the bonds of food molecules CANNOT be used directly by the cell. • Energy from food must be converted to the ONLY energy source that cells can use: ATP! ...
I. B. ATP (adenosine triphosphate) powers cellular work 1. ATP
... • CO2 – more CO2 equals a higher rate only if enough light • 6x CO2 can yield 5x rate of photosynthesis – atmospheric CO2 has increased 10% in last 200 years; not enough to cause great increase in photosynthesis – greenhouse effect- greater impact on length of growing season than rate of photosynthe ...
... • CO2 – more CO2 equals a higher rate only if enough light • 6x CO2 can yield 5x rate of photosynthesis – atmospheric CO2 has increased 10% in last 200 years; not enough to cause great increase in photosynthesis – greenhouse effect- greater impact on length of growing season than rate of photosynthe ...
Chapter 9 Cellular Respiration.notebook
... Most of energy (about 90%) from the glucose is unused and locked into the he electrons of pyruvic acid. To extract this energy the cell uses O2 as a electron acceptor. Oxygen is required for the final steps of cellular respiration. Citric acid is the first compound produced in the Krebs Cycle, so ...
... Most of energy (about 90%) from the glucose is unused and locked into the he electrons of pyruvic acid. To extract this energy the cell uses O2 as a electron acceptor. Oxygen is required for the final steps of cellular respiration. Citric acid is the first compound produced in the Krebs Cycle, so ...
Ch. 9: Cellular Respiration
... A) Aerobic Respiration: Pyruvate is oxidized into carbon dioxide (released) and acetyl-CoA in the Krebs Cycle. Eventually, oxygen gas accepts the high energy H atoms of NADH and FADH2 created in the rest of the Krebs Cycle and water is created as a waste product (electron transport chain in the memb ...
... A) Aerobic Respiration: Pyruvate is oxidized into carbon dioxide (released) and acetyl-CoA in the Krebs Cycle. Eventually, oxygen gas accepts the high energy H atoms of NADH and FADH2 created in the rest of the Krebs Cycle and water is created as a waste product (electron transport chain in the memb ...
Glycolysis Quiz
... glycolysis donates a phosphate group to ADP to form ATP? (a) glucose -6-phosphate (b) PEP (c) PGAL (d) fructose diphosphate ...
... glycolysis donates a phosphate group to ADP to form ATP? (a) glucose -6-phosphate (b) PEP (c) PGAL (d) fructose diphosphate ...
Respiration
... A. Protons are pumped into the intermembrance space B. Electrons are transported across the membrane using ubiquinone (coenzyme Q) and cytochrome C ...
... A. Protons are pumped into the intermembrance space B. Electrons are transported across the membrane using ubiquinone (coenzyme Q) and cytochrome C ...
17 - Doctor Jade Main
... d. a cup of coffee, first thing in the morning. 17. In all cells, the energy used in active transport is provided by a. ATP b. enzymes. c. gravity. d. random molecular motion. 18. The hydrogen atoms of a water molecule are bonded to the oxygen atoms by _______ bonds, whereas neighboring water molecu ...
... d. a cup of coffee, first thing in the morning. 17. In all cells, the energy used in active transport is provided by a. ATP b. enzymes. c. gravity. d. random molecular motion. 18. The hydrogen atoms of a water molecule are bonded to the oxygen atoms by _______ bonds, whereas neighboring water molecu ...
STANDARD 3 EOC 2015
... Standard B- 3: Recognize the overall structure of adenosine triphosphate (ATP)—namely, adenine, the sugar ribose, and three phosphate groups—and summarize its function. Vocabulary: photosynthesis, light-dependent reactions, dark reactions (light-independent reactions), glucose, ATP, ADP, adenine, ri ...
... Standard B- 3: Recognize the overall structure of adenosine triphosphate (ATP)—namely, adenine, the sugar ribose, and three phosphate groups—and summarize its function. Vocabulary: photosynthesis, light-dependent reactions, dark reactions (light-independent reactions), glucose, ATP, ADP, adenine, ri ...
9 and 10 notes with blanks
... A primary electron acceptor in the reaction center accepts excited electrons and is reduced as a result H2O is split by enzymes, and the electrons are transferred from the hydrogen atoms to P680+, thus reducing it to P680 O2 is released as a by-product of this reaction In PS I (like PS II), transfer ...
... A primary electron acceptor in the reaction center accepts excited electrons and is reduced as a result H2O is split by enzymes, and the electrons are transferred from the hydrogen atoms to P680+, thus reducing it to P680 O2 is released as a by-product of this reaction In PS I (like PS II), transfer ...
bio - Gary Brolsma
... Chemiosmosis is the energy coupling mechanism for oxidative phosphorylation and is vital to the synthesis of ATP in mitochondria. It powers most ATP synthesis in cells. This occurs when proteins populating the inner membrane of the mitochondrion called ATP synthase, the enzyme that actually makes AT ...
... Chemiosmosis is the energy coupling mechanism for oxidative phosphorylation and is vital to the synthesis of ATP in mitochondria. It powers most ATP synthesis in cells. This occurs when proteins populating the inner membrane of the mitochondrion called ATP synthase, the enzyme that actually makes AT ...
Fermentation and Cellular Respiration 1. Define: Glycolysis
... glucose. During glycolysis, each glucose molecule is split into two pyruvic acid molecules with the associated production of two molecules of ATP and the reduction of two molecules of NAD to form NADH + H+ (also known as the Embden-Meyerhof-Parnas pathway). Fermentation – Fermentation is the anaerob ...
... glucose. During glycolysis, each glucose molecule is split into two pyruvic acid molecules with the associated production of two molecules of ATP and the reduction of two molecules of NAD to form NADH + H+ (also known as the Embden-Meyerhof-Parnas pathway). Fermentation – Fermentation is the anaerob ...
Cellular Respiration
... Adenosine Tri-Phospate: ◦ Made of three things 1.) Ribose (sugar) 2.) Adenosine (base) 3.) Three phosphates ◦ Key to the activity of ATP ...
... Adenosine Tri-Phospate: ◦ Made of three things 1.) Ribose (sugar) 2.) Adenosine (base) 3.) Three phosphates ◦ Key to the activity of ATP ...
Prof. Kamakaka`s Lecture 10 Notes
... ATP hydrolysis is exogermic (negative DG). This is coupled with endogermic (positive DG) reactions in cells allowing these reactions to proceed. Some processes do involve direct ATP hydrolysis providing energy that changes protein conformation producing mechanical motion ...
... ATP hydrolysis is exogermic (negative DG). This is coupled with endogermic (positive DG) reactions in cells allowing these reactions to proceed. Some processes do involve direct ATP hydrolysis providing energy that changes protein conformation producing mechanical motion ...
Chapter 9: Cellular Respiration
... cycle is stored in the electron carriers NADH and FADH2 2 ATP are produced during the Krebs cycle ...
... cycle is stored in the electron carriers NADH and FADH2 2 ATP are produced during the Krebs cycle ...
Answer Key
... What is the final electron acceptor at the end of Electron Transport? oxygen What happens to the NADH’s produced during glycolysis and Krebs cycle? If oxygen is present, goes to ETC. No oxygen onto fermentation. What high energy electron carriers are used in respiration? NAD+ and FAD How are these d ...
... What is the final electron acceptor at the end of Electron Transport? oxygen What happens to the NADH’s produced during glycolysis and Krebs cycle? If oxygen is present, goes to ETC. No oxygen onto fermentation. What high energy electron carriers are used in respiration? NAD+ and FAD How are these d ...
energy - Wsfcs
... Both photosynthesis and respiration are processes that involve a series of biochemical reactions. Such a series of reactions is called a biochemical pathway. Usable energy produced by one reaction may be stored and used in a later reaction. In most cases this usable energy is stored in a molecule ca ...
... Both photosynthesis and respiration are processes that involve a series of biochemical reactions. Such a series of reactions is called a biochemical pathway. Usable energy produced by one reaction may be stored and used in a later reaction. In most cases this usable energy is stored in a molecule ca ...
Biology 1406 Quiz 2 Multiple-Choice Questions 1) When biologists
... B) It attaches and detaches phosphate groups. C) It uses glucose and generates pyruvate. D) It shifts molecules from cytosol to mitochondrion. E) It uses stored ATP and then forms a net increase in ATP. 45) In cellular respiration, the energy for most ATP synthesis is supplied by A) high energy phos ...
... B) It attaches and detaches phosphate groups. C) It uses glucose and generates pyruvate. D) It shifts molecules from cytosol to mitochondrion. E) It uses stored ATP and then forms a net increase in ATP. 45) In cellular respiration, the energy for most ATP synthesis is supplied by A) high energy phos ...
Cellular Respiration, burning the fuel of life - Jocha
... 6. What are the energy carriers that transport the H atoms and electron from the original glucose to the inside of the mitochondria? In what stages are these produced? 7. In cellular respiration, which stage needs ATP to start the sequence of reactions? 8. Which stages produce ATP, which one produce ...
... 6. What are the energy carriers that transport the H atoms and electron from the original glucose to the inside of the mitochondria? In what stages are these produced? 7. In cellular respiration, which stage needs ATP to start the sequence of reactions? 8. Which stages produce ATP, which one produce ...
Complete breakdown of Glucose:
... DO NOT COPY! This figure won’t be on the exam, I promise! But you still need to know what goes in and what comes out ...
... DO NOT COPY! This figure won’t be on the exam, I promise! But you still need to know what goes in and what comes out ...
File
... Tumor cells have a higher requirement for glucose due to a lower efficiency in energy production from glycolysis. • Complete oxidation of CO2 in healthy cells under aerobic conditions yields ~30 ATP per glucose. • Anaerobic metabolism of glucose in tumor cells yields 2 ATP per glucose. – Glucose tra ...
... Tumor cells have a higher requirement for glucose due to a lower efficiency in energy production from glycolysis. • Complete oxidation of CO2 in healthy cells under aerobic conditions yields ~30 ATP per glucose. • Anaerobic metabolism of glucose in tumor cells yields 2 ATP per glucose. – Glucose tra ...
Biol120 Mock Final Examination (v2.0)
... a) Cellular Respiration products: H2O and CO2 b) Photosynthesis products: O2 and Sugars c) Cellular Respiration reactants: Glucose and H2O d) Photosynthesis reactants: H2O and CO2 7. DNA polymerase works by a) Adding a nucleotide triphosphate to the 3’ end of a DNA primer made by primase. b) Adding ...
... a) Cellular Respiration products: H2O and CO2 b) Photosynthesis products: O2 and Sugars c) Cellular Respiration reactants: Glucose and H2O d) Photosynthesis reactants: H2O and CO2 7. DNA polymerase works by a) Adding a nucleotide triphosphate to the 3’ end of a DNA primer made by primase. b) Adding ...
Bioloical Oxidation - Home
... The free energy made available through the catabolism of fuels (carbohydrates,lipids,amino acids)is not transmitted directly to the reaction requiring energy.Instead it is used to synthesize acompound that acts as acarrier of free energy,which is adenosine triphosphate (ATP). ...
... The free energy made available through the catabolism of fuels (carbohydrates,lipids,amino acids)is not transmitted directly to the reaction requiring energy.Instead it is used to synthesize acompound that acts as acarrier of free energy,which is adenosine triphosphate (ATP). ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.