File
... produce reduced NADH and 4-carbon succinyl-CoA. (One carbon is lost as CO2.) 6. Oxidation of succinyl-CoA produces succinate and one GTP that is converted to ATP. 7. Oxidation of succinate by FAD produces reduced FADH2 and fumarate. 8. Fumarate is converted into malate. 9. Oxidation of malate by NAD ...
... produce reduced NADH and 4-carbon succinyl-CoA. (One carbon is lost as CO2.) 6. Oxidation of succinyl-CoA produces succinate and one GTP that is converted to ATP. 7. Oxidation of succinate by FAD produces reduced FADH2 and fumarate. 8. Fumarate is converted into malate. 9. Oxidation of malate by NAD ...
BSC1010 Quiz 2 Answers - Palm Beach State College
... 29) In chemiosmosis, what is the most direct source of energy that is used to convert ADP + i to ATP? A) energy released as electrons flow through the electron transport system B) energy released from substrate-level phosphorylation C) energy released from movement of protons through ATP synthase, ...
... 29) In chemiosmosis, what is the most direct source of energy that is used to convert ADP + i to ATP? A) energy released as electrons flow through the electron transport system B) energy released from substrate-level phosphorylation C) energy released from movement of protons through ATP synthase, ...
PowerPoint Presentation - Ch. 6 Cellular Respiration
... Stored as a polysaccharide, such as glycogen, in our liver & muscle cells. How is glycogen used between meals? Glycogen is hydrolyzed to glucose to serve as fuel between meals. ...
... Stored as a polysaccharide, such as glycogen, in our liver & muscle cells. How is glycogen used between meals? Glycogen is hydrolyzed to glucose to serve as fuel between meals. ...
A chemist has discovered a drug that blocks
... 3. How do you account for a situation in which a person can utilize only fatty acids and amino acids, resulting in the production of more lactate than normal? The affected person's cells: a. have mitochondria lacking the transport protein that moves pyruvate across the outer mitochondrial membrane. ...
... 3. How do you account for a situation in which a person can utilize only fatty acids and amino acids, resulting in the production of more lactate than normal? The affected person's cells: a. have mitochondria lacking the transport protein that moves pyruvate across the outer mitochondrial membrane. ...
Title - Iowa State University
... 11. Which of the following best describes the process that occurs during the citric acid cycle? a. Pyruvate is processed to release one molecule of carbon dioxide, and the remaining carbons are used to form acetyl CoA. b. One molecule of glucose is broken into two molecules of pyruvate, ATP is produ ...
... 11. Which of the following best describes the process that occurs during the citric acid cycle? a. Pyruvate is processed to release one molecule of carbon dioxide, and the remaining carbons are used to form acetyl CoA. b. One molecule of glucose is broken into two molecules of pyruvate, ATP is produ ...
Syllabus for BASIC METABOLIC PRINCIPLES
... Thioesters (red box in figure), especially those involving Coenzyme A, are very important coenzymes that participate in transfer of acyl and acetyl groups from glycolysis to the Citric Acid Cycle and to the fatty acid synthesis pathway. The sulfhydral group on CoA reacts ...
... Thioesters (red box in figure), especially those involving Coenzyme A, are very important coenzymes that participate in transfer of acyl and acetyl groups from glycolysis to the Citric Acid Cycle and to the fatty acid synthesis pathway. The sulfhydral group on CoA reacts ...
Sample exam 1
... 6. Gastric juice has a pH of 1.5 and is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. a. Calculate the free energy required to concentrate the H+ in 1 L of gastric juice at 37°C. For this problem, you can ignore the effects of the transmembrane electrical potential difference. ...
... 6. Gastric juice has a pH of 1.5 and is produced by pumping HCl from blood plasma (pH 7.4) into the stomach. a. Calculate the free energy required to concentrate the H+ in 1 L of gastric juice at 37°C. For this problem, you can ignore the effects of the transmembrane electrical potential difference. ...
Answer Key 2016 Spring Biology (General) Exam #2
... 19)Which statement correctly describes carbon fixation? A) the conversion of CO2 to an organic compound B) the reduction of a high energy electron carrier to a low energy carrier C) the production of carbohydrate molecules from the 3-carbon compound G3P D) the use of ATP and NADPH to reduce CO2 20) ...
... 19)Which statement correctly describes carbon fixation? A) the conversion of CO2 to an organic compound B) the reduction of a high energy electron carrier to a low energy carrier C) the production of carbohydrate molecules from the 3-carbon compound G3P D) the use of ATP and NADPH to reduce CO2 20) ...
Cellular respiration
... Although there is a theoretical yield of 38 ATP molecules per glucose during cellular respiration, such conditions are generally not realized due to losses such as the cost of moving pyruvate (from glycolysis), phosphate, and ADP (substrates for ATP synthesis) into the mitochondria. All are actively ...
... Although there is a theoretical yield of 38 ATP molecules per glucose during cellular respiration, such conditions are generally not realized due to losses such as the cost of moving pyruvate (from glycolysis), phosphate, and ADP (substrates for ATP synthesis) into the mitochondria. All are actively ...
Cellular Respiration
... • Each NADH contributes enough E to generate a maximum of 3 ATP • In some eukaryotic cells, NADH produced in the cytosol by glycolysis may only be worth 2 ATP – The e-s must be shuttled to the mitochondrion – In some shuttle systems, the e-s are passed to NAD+, in others the e-s are passed to FAD • ...
... • Each NADH contributes enough E to generate a maximum of 3 ATP • In some eukaryotic cells, NADH produced in the cytosol by glycolysis may only be worth 2 ATP – The e-s must be shuttled to the mitochondrion – In some shuttle systems, the e-s are passed to NAD+, in others the e-s are passed to FAD • ...
Cellular Respiration
... • Each NADH contributes enough E to generate a maximum of 3 ATP • In some eukaryotic cells, NADH produced in the cytosol by glycolysis may only be worth 2 ATP – The e-s must be shuttled to the mitochondrion – In some shuttle systems, the e-s are passed to NAD+, in others the e-s are passed to FAD • ...
... • Each NADH contributes enough E to generate a maximum of 3 ATP • In some eukaryotic cells, NADH produced in the cytosol by glycolysis may only be worth 2 ATP – The e-s must be shuttled to the mitochondrion – In some shuttle systems, the e-s are passed to NAD+, in others the e-s are passed to FAD • ...
Macronutrients
... Is a series of three reactions: 1. Glycolysis 2. Krebs Cycle 3. Electron Transport Chain ...
... Is a series of three reactions: 1. Glycolysis 2. Krebs Cycle 3. Electron Transport Chain ...
Ch 9 Notes Cellular Respiration: Harvesting Chemical Energy
... it’s a series of reactions that remove electrons from the sugar (what’s left of them). We are now entering the mitochondria Goes in: Pyruvate (converted to Acetyl Co-A), NAD+, FAD. Comes out: CO2 , 6NADH, 2FADH2, 2ATP ...
... it’s a series of reactions that remove electrons from the sugar (what’s left of them). We are now entering the mitochondria Goes in: Pyruvate (converted to Acetyl Co-A), NAD+, FAD. Comes out: CO2 , 6NADH, 2FADH2, 2ATP ...
Chapter 9: How do cells harvest energy?
... process at least some of the time; also called cellular respiration (How is this different from breathing, and how is it related to breathing?) B. anaerobic respiration – processes similar to aerobic respiration but that do not use O 2; used mainly by bacteria that live in ...
... process at least some of the time; also called cellular respiration (How is this different from breathing, and how is it related to breathing?) B. anaerobic respiration – processes similar to aerobic respiration but that do not use O 2; used mainly by bacteria that live in ...
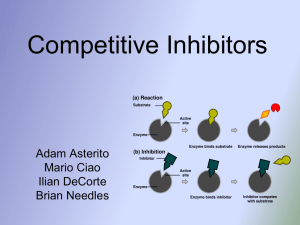
Competitive Inhibitors
... at the active site, where the substrates would normally bind. • This protein has a similar shape of the substrate that binds to the enzyme, it will cause the enzyme to stop working (inhibit). • An inhibitor can be reversible if the concentration of the substrate is increased enough. ...
... at the active site, where the substrates would normally bind. • This protein has a similar shape of the substrate that binds to the enzyme, it will cause the enzyme to stop working (inhibit). • An inhibitor can be reversible if the concentration of the substrate is increased enough. ...
BIE 5810 - Chapter 5, Part I
... (2) Efficiency in utilizing total energy potentially available from glucose: E= 14,600 cal__ = 2% (typical of fermentations) 686,.000 cal 1. (p. 139) TCA cycle main functions: 1. provide e (NADH) for electron transport chain and biosynthesis 2. supply C skeletons for AMINO ACID synthesis 3. generate ...
... (2) Efficiency in utilizing total energy potentially available from glucose: E= 14,600 cal__ = 2% (typical of fermentations) 686,.000 cal 1. (p. 139) TCA cycle main functions: 1. provide e (NADH) for electron transport chain and biosynthesis 2. supply C skeletons for AMINO ACID synthesis 3. generate ...
Photosynthesis
... Cellular Respiration – chemical energy from glucose and other food is released in a chemical pathway to control the speed and amount • includes all the chemical reactions in which energy is released in support of cell life ...
... Cellular Respiration – chemical energy from glucose and other food is released in a chemical pathway to control the speed and amount • includes all the chemical reactions in which energy is released in support of cell life ...
Bio 6B Lecture Slides - R1
... Pyruvate / H+ symporter Proton gradient drives cotransport of pyruvate & H+ into matrix Pyruvate H+ ...
... Pyruvate / H+ symporter Proton gradient drives cotransport of pyruvate & H+ into matrix Pyruvate H+ ...
Mitochondrial Mechanisms of Photobiomodulation in Context of New
... the cellular metabolism, especially in suppressed or otherwise ill cells.7,8 In connection with the versatility of LLLT effects, I draw the readers’ attention to a comparatively new aspect of the ATP molecule. A long series of discoveries has demonstrated that ATP is not only an energy currency insi ...
... the cellular metabolism, especially in suppressed or otherwise ill cells.7,8 In connection with the versatility of LLLT effects, I draw the readers’ attention to a comparatively new aspect of the ATP molecule. A long series of discoveries has demonstrated that ATP is not only an energy currency insi ...
3 biochemistry, macromolecules
... • If no oxygen is available pyruvic acid is converted to lactic acid (build up causes muscle soreness) • No ATP produced • Allows glycolysis to start over (regenerates NAD+) ...
... • If no oxygen is available pyruvic acid is converted to lactic acid (build up causes muscle soreness) • No ATP produced • Allows glycolysis to start over (regenerates NAD+) ...
Chapter 9 - H-W Science Website
... 4. Explain in general terms how redox reactions are involved in energy exchanges. 5. Describe the role of NAD+ and FAD in cellular respiration. 6. In general terms, explain the role of the electron transport chain in cellular respiration. 7. Name the three stages of cellular respiration and state th ...
... 4. Explain in general terms how redox reactions are involved in energy exchanges. 5. Describe the role of NAD+ and FAD in cellular respiration. 6. In general terms, explain the role of the electron transport chain in cellular respiration. 7. Name the three stages of cellular respiration and state th ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.