Cellular Respiration
... – An oxidized gram of fat produces >2X ATP as oxidized gram of carbohydrate ...
... – An oxidized gram of fat produces >2X ATP as oxidized gram of carbohydrate ...
Chapter 8
... transferring a phosphate directly to ADP from another molecule 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP ...
... transferring a phosphate directly to ADP from another molecule 2. oxidative phosphorylation – use of ATP synthase and energy derived from a proton (H+) gradient to make ATP ...
Oxidative Phosphorylation
... The third complex is composed of cytochrome b, another Fe-S protein, Rieske center (2Fe-2S center), and cytochrome c proteins; this complex is also called cytochrome oxidoreductase. Cytochrome proteins have a prosthetic group of heme. The heme molecule is similar to the heme in hemoglobin, but it ca ...
... The third complex is composed of cytochrome b, another Fe-S protein, Rieske center (2Fe-2S center), and cytochrome c proteins; this complex is also called cytochrome oxidoreductase. Cytochrome proteins have a prosthetic group of heme. The heme molecule is similar to the heme in hemoglobin, but it ca ...
Chapter 9 review sheet
... phosphorylation, electron carriers, heme, cytochrome c, ubiquinone, complex I, complex II, complex III, complex IV, mitochondria, NAD+, NADH, citrate, citrate synthase, hexokinase, phosphofructokinase, G3P, FAD, FADH2, glycolysis, glucose, cytosol, inner mitochondrial membrane, outer mitochondrial m ...
... phosphorylation, electron carriers, heme, cytochrome c, ubiquinone, complex I, complex II, complex III, complex IV, mitochondria, NAD+, NADH, citrate, citrate synthase, hexokinase, phosphofructokinase, G3P, FAD, FADH2, glycolysis, glucose, cytosol, inner mitochondrial membrane, outer mitochondrial m ...
FERMENTATION: an anaerobic biological reaction process in which
... glycolysis, fatty acid breakdown, the Krebs (citric acid cycle) and electron transport As a result of many control mechanisms, the body oxidizes fats and sugars 5-10 times more rapidly during a period of strenuous exercise than during a period of rest ...
... glycolysis, fatty acid breakdown, the Krebs (citric acid cycle) and electron transport As a result of many control mechanisms, the body oxidizes fats and sugars 5-10 times more rapidly during a period of strenuous exercise than during a period of rest ...
biol 161 aerobic cellular respiration
... 1. Where are the protein carriers of the electron transport chain located? 2. As electrons are passed along the electron transport chain, the energy generated pumps hydrogen ions from the ____________ to the ___________________ of a mitochondrion so that an electrochemical gradient of H+ is establis ...
... 1. Where are the protein carriers of the electron transport chain located? 2. As electrons are passed along the electron transport chain, the energy generated pumps hydrogen ions from the ____________ to the ___________________ of a mitochondrion so that an electrochemical gradient of H+ is establis ...
Review Guide for Third Exam in Biochemistry 507 (1997)
... reaction itself, the way in which branches are degraded. 3. Be ready to explain the requirement for Mg2+ ion in glycolysis reactions. 4. The first and second stages of glycolysis: where is ATP invested, where is it gained, and how much net gain is there? 5. Regulation of PFK-1: what are the regulato ...
... reaction itself, the way in which branches are degraded. 3. Be ready to explain the requirement for Mg2+ ion in glycolysis reactions. 4. The first and second stages of glycolysis: where is ATP invested, where is it gained, and how much net gain is there? 5. Regulation of PFK-1: what are the regulato ...
The Citric Acid Cycle
... Acetyl-CoA + 3NAD+ + FAD + GDP + Pi + 2 H2O 2CO2 +3NADH + FADH2 + GTP + CoA + 3H+ • Carbons of acetyl groups in acetyl-CoA are oxidized to CO2 • Electrons from this process reduce NAD+ and FAD • One GTP is formed per cycle, this can be converted to ATP • Intermediates in the cycle are not depleted ...
... Acetyl-CoA + 3NAD+ + FAD + GDP + Pi + 2 H2O 2CO2 +3NADH + FADH2 + GTP + CoA + 3H+ • Carbons of acetyl groups in acetyl-CoA are oxidized to CO2 • Electrons from this process reduce NAD+ and FAD • One GTP is formed per cycle, this can be converted to ATP • Intermediates in the cycle are not depleted ...
Slide 1
... ATP is the end product of glycolysis as well as it is substrate for PFK-1. In presence of high concentration of ATP, ATP binds to inhibition site of PFK, and thereby decreases the activity of enzyme. AMP, ADP and Fructose 2, 6 biphosphate act as allosteric activators of this enzyme. Activation of en ...
... ATP is the end product of glycolysis as well as it is substrate for PFK-1. In presence of high concentration of ATP, ATP binds to inhibition site of PFK, and thereby decreases the activity of enzyme. AMP, ADP and Fructose 2, 6 biphosphate act as allosteric activators of this enzyme. Activation of en ...
Cellular Respiration
... • Electron-carrying redox molecules (NADH and FADH2) transfer their electrons to the e- transport chain • The e- transport chain uses the electrons to create a proton gradient across the inner mitochondrial membrane • ATP synthase uses the potential energy in the proton gradient to convert much ADP ...
... • Electron-carrying redox molecules (NADH and FADH2) transfer their electrons to the e- transport chain • The e- transport chain uses the electrons to create a proton gradient across the inner mitochondrial membrane • ATP synthase uses the potential energy in the proton gradient to convert much ADP ...
Lecture 16 (Parker) - Department of Chemistry ::: CALTECH
... Electron transport and ATP synthesis are coupled by a proton gradient across the inner mitochondrial membrane. In this model the transfer of electrons through the respiratory chain leads to the pumping of protons from the matrix to the cytoplasmic side of the inner mitochondrial membrane. ATP syntha ...
... Electron transport and ATP synthesis are coupled by a proton gradient across the inner mitochondrial membrane. In this model the transfer of electrons through the respiratory chain leads to the pumping of protons from the matrix to the cytoplasmic side of the inner mitochondrial membrane. ATP syntha ...
Communication
... recall the structure of a liver mitochondrion identify inner and outer membranes and the inter membranal space state that, during aerobic respiration in animals, pyruvate is actively transported into mitochondria; explain, with the aid of diagrams and electron micrographs, how the structure of ...
... recall the structure of a liver mitochondrion identify inner and outer membranes and the inter membranal space state that, during aerobic respiration in animals, pyruvate is actively transported into mitochondria; explain, with the aid of diagrams and electron micrographs, how the structure of ...
Ch2
... Do not start chemical reactions or set ATP yield Do facilitate breakdown (catabolism) of substrates Lower the activation energy for a chemical reaction End with suffix -ase ...
... Do not start chemical reactions or set ATP yield Do facilitate breakdown (catabolism) of substrates Lower the activation energy for a chemical reaction End with suffix -ase ...
2 Pyruvic Acid
... During respiration electrons are removed from glucose and transported to the ETC by electron carriers. Energy from the electrons is used to synthesize ATP in the ETC. ...
... During respiration electrons are removed from glucose and transported to the ETC by electron carriers. Energy from the electrons is used to synthesize ATP in the ETC. ...
study guide
... Chemical Energy and ATP Energy is the ability to do work. Organisms need energy to stay alive. Adenosine triphosphate (ATP) is a chemical compound cells use to store and release energy. An ATP molecule consists of adenine, the sugar ribose, and three phosphate groups. Cells store energy by addin ...
... Chemical Energy and ATP Energy is the ability to do work. Organisms need energy to stay alive. Adenosine triphosphate (ATP) is a chemical compound cells use to store and release energy. An ATP molecule consists of adenine, the sugar ribose, and three phosphate groups. Cells store energy by addin ...
basic biochemistry - Personal Webspace for QMUL
... The loss of the phosphate creates pyruvate in an unstable enol form The free-energy released on the rearrangement of pyruvate to its more stable ketone form is more than is needed to produce ATP REGULATION OF GLYCOLYSIS Glycolysis regulation reflects its dual role in: Degrading glucose to ...
... The loss of the phosphate creates pyruvate in an unstable enol form The free-energy released on the rearrangement of pyruvate to its more stable ketone form is more than is needed to produce ATP REGULATION OF GLYCOLYSIS Glycolysis regulation reflects its dual role in: Degrading glucose to ...
Electron transport chain
... • If O2 is not available to the cell, fermentation, an anaerobic process, occurs in the cytoplasm. During fermentation, glucose is incompletely metabolized to lactate, or to CO2 and alcohol (depending on the organism). ...
... • If O2 is not available to the cell, fermentation, an anaerobic process, occurs in the cytoplasm. During fermentation, glucose is incompletely metabolized to lactate, or to CO2 and alcohol (depending on the organism). ...
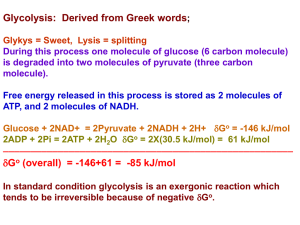
Glycolysis [Compatibility Mode]
... two molecules of a three-carbon sugar. Glycolysis yields two molecules of ATP Glycolysis does not require oxygen It is also known as anaerobic cellular respiration ...
... two molecules of a three-carbon sugar. Glycolysis yields two molecules of ATP Glycolysis does not require oxygen It is also known as anaerobic cellular respiration ...
Lecture Resource ()
... In each of these transformations, one of the bonds to the a-carbon of the amino acid substrate is broken in the first step of the reaction ...
... In each of these transformations, one of the bonds to the a-carbon of the amino acid substrate is broken in the first step of the reaction ...
Practice photosynthesis/Respiration
... D) oxygen, carbon dioxide, and water E) NADH, FADH2 , and electrons 33) Which of the following most accurately describes what is happening along this chain? A) Each electron carrier alternates between being reduced and being oxidized. B) Energy of the electrons increases at each step. C) Molecules i ...
... D) oxygen, carbon dioxide, and water E) NADH, FADH2 , and electrons 33) Which of the following most accurately describes what is happening along this chain? A) Each electron carrier alternates between being reduced and being oxidized. B) Energy of the electrons increases at each step. C) Molecules i ...
Cellular Respiration
... convert the pyruvate from glycolysis into lactic acid. Skeletal muscles in the body produce lactic acid during strenuous exercise, such as weight lifting and long distance running. When lactic acids levels build up, the muscles feel fatigued and sore. Lactic acid fermentation also occurs in certain ...
... convert the pyruvate from glycolysis into lactic acid. Skeletal muscles in the body produce lactic acid during strenuous exercise, such as weight lifting and long distance running. When lactic acids levels build up, the muscles feel fatigued and sore. Lactic acid fermentation also occurs in certain ...
Big Idea #2
... of pyruvate, 2 ATP, and 2 NADH Pyruvate oxidation: two pyruvates enter the mitochondrial matrix. They are converted to 2 Acetyl CoA which go on to the Krebs Cycles; and 2 NADH which go on to oxidative phosphorylation. Krebs Cycle: Occurs in mitochondrial matrix. Two turns of the cycle convert th ...
... of pyruvate, 2 ATP, and 2 NADH Pyruvate oxidation: two pyruvates enter the mitochondrial matrix. They are converted to 2 Acetyl CoA which go on to the Krebs Cycles; and 2 NADH which go on to oxidative phosphorylation. Krebs Cycle: Occurs in mitochondrial matrix. Two turns of the cycle convert th ...
12.3 The Citric Acid Cycle Oxidizes AcetylCoA
... CoA to CO2 by NAD+ and Q • The cycle itself is not a pathway for a net degradation of any cycle intermediates • Cycle intermediates can be shared with other pathways, which may lead to a resupply or net decrease in cycle intermediates ...
... CoA to CO2 by NAD+ and Q • The cycle itself is not a pathway for a net degradation of any cycle intermediates • Cycle intermediates can be shared with other pathways, which may lead to a resupply or net decrease in cycle intermediates ...
Adenosine triphosphate
Adenosine triphosphate (ATP) is a nucleoside triphosphate used in cells as a coenzyme often called the ""molecular unit of currency"" of intracellular energy transfer.ATP transports chemical energy within cells for metabolism. It is one of the end products of photophosphorylation, cellular respiration, and fermentation and used by enzymes and structural proteins in many cellular processes, including biosynthetic reactions, motility, and cell division. One molecule of ATP contains three phosphate groups, and it is produced by a wide variety of enzymes, including ATP synthase, from adenosine diphosphate (ADP) or adenosine monophosphate (AMP) and various phosphate group donors. Substrate-level phosphorylation, oxidative phosphorylation in cellular respiration, and photophosphorylation in photosynthesis are three major mechanisms of ATP biosynthesis.Metabolic processes that use ATP as an energy source convert it back into its precursors. ATP is therefore continuously recycled in organisms: the human body, which on average contains only 250 grams (8.8 oz) of ATP, turns over its own body weight equivalent in ATP each day.ATP is used as a substrate in signal transduction pathways by kinases that phosphorylate proteins and lipids. It is also used by adenylate cyclase, which uses ATP to produce the second messenger molecule cyclic AMP. The ratio between ATP and AMP is used as a way for a cell to sense how much energy is available and control the metabolic pathways that produce and consume ATP. Apart from its roles in signaling and energy metabolism, ATP is also incorporated into nucleic acids by polymerases in the process of transcription. ATP is the neurotransmitter believed to signal the sense of taste.The structure of this molecule consists of a purine base (adenine) attached by the 9' nitrogen atom to the 1' carbon atom of a pentose sugar (ribose). Three phosphate groups are attached at the 5' carbon atom of the pentose sugar. It is the addition and removal of these phosphate groups that inter-convert ATP, ADP and AMP. When ATP is used in DNA synthesis, the ribose sugar is first converted to deoxyribose by ribonucleotide reductase.ATP was discovered in 1929 by Karl Lohmann, and independently by Cyrus Fiske and Yellapragada Subbarow of Harvard Medical School, but its correct structure was not determined until some years later. It was proposed to be the intermediary molecule between energy-yielding and energy-requiring reactions in cells by Fritz Albert Lipmann in 1941. It was first artificially synthesized by Alexander Todd in 1948.

















![Glycolysis [Compatibility Mode]](http://s1.studyres.com/store/data/015804080_1-177318d0ac1e37e7516326c4d927443b-300x300.png)