CHAPTER 6
... regulated by covalent modification. ADPribosylation of nitrogenase reductase leads to its ...
... regulated by covalent modification. ADPribosylation of nitrogenase reductase leads to its ...
HIV Protease Inhibitor: Past Endeavors and Future Developments
... with the actual proteins. Containing these analogues, many inhibitors have diverse chemical backbones such as hydroxyaminopentane amides, hydroxyethylamino sulfonamides (141W94), allophenylnorstatines (KNI-272), cyclic sulfones (GS-3333), irreversible-binding cis-epoxide compounds (LB-71148) and LB- ...
... with the actual proteins. Containing these analogues, many inhibitors have diverse chemical backbones such as hydroxyaminopentane amides, hydroxyethylamino sulfonamides (141W94), allophenylnorstatines (KNI-272), cyclic sulfones (GS-3333), irreversible-binding cis-epoxide compounds (LB-71148) and LB- ...
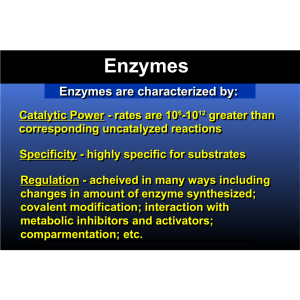
Enzymes
... ***Enzymes are classified as catalysts. Catalysts are substances that increase the rate of a reaction, but are not changed. ...
... ***Enzymes are classified as catalysts. Catalysts are substances that increase the rate of a reaction, but are not changed. ...
Studies on the key amino acid residues responsible for the alkali
... pH optimum of the xylanase and a decrease in its activity by ∼20%. All of these suggested that Asn-71 might be involved in the catalytic active center and was critical for the alkaline tolerance of the xylanase. In order to search other residues of the xylanase besides Asn-71, which are involved in ...
... pH optimum of the xylanase and a decrease in its activity by ∼20%. All of these suggested that Asn-71 might be involved in the catalytic active center and was critical for the alkaline tolerance of the xylanase. In order to search other residues of the xylanase besides Asn-71, which are involved in ...
Lecture Topic: Fatty Acid Synthesis
... Synthesis of the Amino Acids Amino acids differ from other biomolecules in that each member is synthesized in a unique ...
... Synthesis of the Amino Acids Amino acids differ from other biomolecules in that each member is synthesized in a unique ...
Biochemical studies on a versatile esterase that is most catalytically
... similarity: 68%). It was also homologous (≤ 56% identity) to predicted esterases/lipases from uncultured microorganisms derived from soil and marine sediment samples (Fig. 3). This enzyme can be categorized in the microbial family IV described by Arpigny and Jaeger (1999); it includes a typical -Gly ...
... similarity: 68%). It was also homologous (≤ 56% identity) to predicted esterases/lipases from uncultured microorganisms derived from soil and marine sediment samples (Fig. 3). This enzyme can be categorized in the microbial family IV described by Arpigny and Jaeger (1999); it includes a typical -Gly ...
Fundementals I
... important are the first amino group of the first amino acid and the carboxyl group of the last amino acid. The more basic side-chains: Lysine (Lys, K) pKa=10.5 Serine (Ser, S) pKa=13 Threonine (Thr, T) pKa=13 Tyrosine (Tyr, Y) pKa=10.1 *Would you expect Serine to ever have an ionization in a physiol ...
... important are the first amino group of the first amino acid and the carboxyl group of the last amino acid. The more basic side-chains: Lysine (Lys, K) pKa=10.5 Serine (Ser, S) pKa=13 Threonine (Thr, T) pKa=13 Tyrosine (Tyr, Y) pKa=10.1 *Would you expect Serine to ever have an ionization in a physiol ...
This exam has 8 pages, including this one.
... except for Tyrosine. Provide the name for each amino acid that you have drawn (6 pts). ii) Indicate, on the polar amino acid, how a single water molecule would hydrogen bond to one of its sidechain atoms. Indicate both the donor and acceptor for this interaction. Also indicate the approximate length ...
... except for Tyrosine. Provide the name for each amino acid that you have drawn (6 pts). ii) Indicate, on the polar amino acid, how a single water molecule would hydrogen bond to one of its sidechain atoms. Indicate both the donor and acceptor for this interaction. Also indicate the approximate length ...
Lecture 27
... Glu synthesized by Glutamate synthase. Occurs only in microorganisms, plants, and lower animals. Converts -ketoglutarate and ammonia from glutamine to glutamate. Reductive amination requires electrons from either NADPH or ferredoxin (organism dependent). NADPH-dependent glutamine synthase from Azos ...
... Glu synthesized by Glutamate synthase. Occurs only in microorganisms, plants, and lower animals. Converts -ketoglutarate and ammonia from glutamine to glutamate. Reductive amination requires electrons from either NADPH or ferredoxin (organism dependent). NADPH-dependent glutamine synthase from Azos ...
Regulation of Glycolysis
... Because the principle function of glycolysis is to produce ATP, it must be regulated so that ATP is generated only when needed. The enzyme which controls the flux of metabolites through the glycolytic pathway is phosphofructokinase (PFK-1). PFK-1 is an allosteric enzyme that occupies the key regulat ...
... Because the principle function of glycolysis is to produce ATP, it must be regulated so that ATP is generated only when needed. The enzyme which controls the flux of metabolites through the glycolytic pathway is phosphofructokinase (PFK-1). PFK-1 is an allosteric enzyme that occupies the key regulat ...
College Accounting: A Practical Approach, Cdn
... Gly-Ser-Ala-His-Phe-Pro-Asn-Ala-Val-Glu-Cys-Ala-Ser A) chymotrypsin and thrombin B) trypsin and chymotrypsin C) thermolysin and chymotrypsin D) trypsin and thermolysin E) none of the above Answer: C Difficulty: 2 ...
... Gly-Ser-Ala-His-Phe-Pro-Asn-Ala-Val-Glu-Cys-Ala-Ser A) chymotrypsin and thrombin B) trypsin and chymotrypsin C) thermolysin and chymotrypsin D) trypsin and thermolysin E) none of the above Answer: C Difficulty: 2 ...
Notes - Part 2.
... The actin-dependent ATP-hydrolyase (ATPase) activity is localised in the globular heads of myosin (labelled S1) each of which also binds two different "light chains". The enzyme-catalysed reaction involves a profound conformational change in the structure of S1. It was one of the first enzymes for w ...
... The actin-dependent ATP-hydrolyase (ATPase) activity is localised in the globular heads of myosin (labelled S1) each of which also binds two different "light chains". The enzyme-catalysed reaction involves a profound conformational change in the structure of S1. It was one of the first enzymes for w ...
Enzymes - Food Science & Human Nutrition
... ◦ All enzymes have a certain narrow range of pH where they perform best Most active between 4.5-8 Some active at very low (e.g. pepsin) or high pH ...
... ◦ All enzymes have a certain narrow range of pH where they perform best Most active between 4.5-8 Some active at very low (e.g. pepsin) or high pH ...
Catalytic triad

A catalytic triad refers to the three amino acid residues that function together at the centre of the active site of some hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, acylases, lipases and β-lactamases). An Acid-Base-Nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine. Because enzymes fold into complex three-dimensional structures, the residues of a catalytic triad can be far from each other along the amino-acid sequence (primary structure), however, they are brought close together in the final fold.As well as divergent evolution of function (and even the triad's nucleophile), catalytic triads show some of the best examples of convergent evolution. Chemical constraints on catalysis have led to the same catalytic solution independently evolving in at least 23 separate superfamilies. Their mechanism of action is consequently one of the best studied in biochemistry.