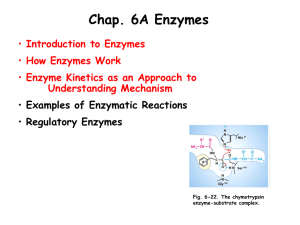
allyl cysteine sulphoxide
... callus have been maintained for up to 15 days on a phytogel/MS medium, with and without sulphate, containing a range of potential precursors to the synthesis of Alliin (at different concentrations). This method of substrate feeding will only give a positive result if: the substrate gets into the ...
... callus have been maintained for up to 15 days on a phytogel/MS medium, with and without sulphate, containing a range of potential precursors to the synthesis of Alliin (at different concentrations). This method of substrate feeding will only give a positive result if: the substrate gets into the ...
Lehninger Principles of Biochemistry
... constituent reactant species, thus slowing the reaction (Fig. 6-8). Charged intermediates can often be stabilized by the transfer of protons to or from the substrate or intermediate to form a species that breaks down more readily to products. Catalysis, such as in organic chemistry reactions, that u ...
... constituent reactant species, thus slowing the reaction (Fig. 6-8). Charged intermediates can often be stabilized by the transfer of protons to or from the substrate or intermediate to form a species that breaks down more readily to products. Catalysis, such as in organic chemistry reactions, that u ...
amino-terminal
... of amino acids in a protein • We can calculate the approximate number of amino • acid residues in a simple protein containing no other chemical constituents by dividing its molecular weight by 110. • Although the average molecular weight of the 20 common amino acids is about 138, the smaller amino a ...
... of amino acids in a protein • We can calculate the approximate number of amino • acid residues in a simple protein containing no other chemical constituents by dividing its molecular weight by 110. • Although the average molecular weight of the 20 common amino acids is about 138, the smaller amino a ...
DNACatalyzed Lysine Side Chain Modification
... achieved by using 5’-ImpDNA in the less preorganized architecture. From these results, we conclude that DNA can catalyze covalent modification of the nucleophilic Lys side chain, and a high degree of preorganization is dispensable when the electrophile is sufficiently reactive. In vitro selection[9] ...
... achieved by using 5’-ImpDNA in the less preorganized architecture. From these results, we conclude that DNA can catalyze covalent modification of the nucleophilic Lys side chain, and a high degree of preorganization is dispensable when the electrophile is sufficiently reactive. In vitro selection[9] ...
View document as PDF
... This collection is designed to introduce students to the concepts of protein structure and biochemistry. Different activities guide students from the basic building blocks of proteins, amino acids, through the different levels of protein structure. Using the MolyMod© models, students learn the diffe ...
... This collection is designed to introduce students to the concepts of protein structure and biochemistry. Different activities guide students from the basic building blocks of proteins, amino acids, through the different levels of protein structure. Using the MolyMod© models, students learn the diffe ...
Metabolism of amino acids
... a) tyrosine → serotonin b) serine → ethanolamine c) tryptophan → catecholamines d) cysteine → taurine ...
... a) tyrosine → serotonin b) serine → ethanolamine c) tryptophan → catecholamines d) cysteine → taurine ...
Slide 1
... •Ankyrin Repeats Domain (ARD) monomeric in solution. •Ankyrin repeats are approximately 33 amino acid residues in length consisting of two anti-parallel alpha helices separated by intervening finger loop regions. •The three-dimensional structure of TRPV2-ARD consists of 6 ankyrin repeat structure mo ...
... •Ankyrin Repeats Domain (ARD) monomeric in solution. •Ankyrin repeats are approximately 33 amino acid residues in length consisting of two anti-parallel alpha helices separated by intervening finger loop regions. •The three-dimensional structure of TRPV2-ARD consists of 6 ankyrin repeat structure mo ...
Reduction of Feedback Inhibition in Homoserine
... L-threonine is a limiting amino acid in livestock diets. Currently, L-threonine is produced by E. coli, which makes the purification of L-threonine difficult because it produces endotoxins. Thus, we seek to overproduce L-threonine by using Corynebacterium glutamicum, a GRAS (generally regarded as sa ...
... L-threonine is a limiting amino acid in livestock diets. Currently, L-threonine is produced by E. coli, which makes the purification of L-threonine difficult because it produces endotoxins. Thus, we seek to overproduce L-threonine by using Corynebacterium glutamicum, a GRAS (generally regarded as sa ...
Biomolecules
... All biologically known protein are polymers of a set of twenty known amino acids. All biologically known amino acids are α L amino acids. They are composed of carboxylic end COOH and amino end NH2 and α carbon attached to both of them and special side chain (R) attached to this α carbon . This side ...
... All biologically known protein are polymers of a set of twenty known amino acids. All biologically known amino acids are α L amino acids. They are composed of carboxylic end COOH and amino end NH2 and α carbon attached to both of them and special side chain (R) attached to this α carbon . This side ...
lecture notes
... The Alpha Helix3 There was ample evidence prior to Pauling’s work that peptides can form helical structures. However, Pauling recognized both the relevance of the hydrogen bond in shaping molecular structure and appreciated the conformational restraints associated with the peptide bond. Guided by a ...
... The Alpha Helix3 There was ample evidence prior to Pauling’s work that peptides can form helical structures. However, Pauling recognized both the relevance of the hydrogen bond in shaping molecular structure and appreciated the conformational restraints associated with the peptide bond. Guided by a ...
Lecture #1 ~ Date_________
... • Inhibitor binds to site other than active site – allosteric inhibitor binds to allosteric site – causes enzyme to change shape • conformational change • active site is no longer functional binding site – keeps enzyme inactive ...
... • Inhibitor binds to site other than active site – allosteric inhibitor binds to allosteric site – causes enzyme to change shape • conformational change • active site is no longer functional binding site – keeps enzyme inactive ...
Slide 1
... of substrate) with the enzyme but is not transformed into product/s and thus blocks the active site temporarily or permanently is called • A.Co-enzyme B.Blocker C.Inhibitor 10 The structure of an enzyme is altered by: • A.Irreversible inhibitor B.Reversible inhibitor ...
... of substrate) with the enzyme but is not transformed into product/s and thus blocks the active site temporarily or permanently is called • A.Co-enzyme B.Blocker C.Inhibitor 10 The structure of an enzyme is altered by: • A.Irreversible inhibitor B.Reversible inhibitor ...
AMINO ACIDS METABOLISM ** Dr. Mohammed Abdullateef **
... the blood and other biological fluids → ammonia difuses into cells, across blood/brain barrier → increased synthesis of glutamate from a-ketoglutarate by glutamate dehydrogenase, increased synthesis of glutamine. Alpha ketoglutarate is consumed and not available to reach other processes such as TCA ...
... the blood and other biological fluids → ammonia difuses into cells, across blood/brain barrier → increased synthesis of glutamate from a-ketoglutarate by glutamate dehydrogenase, increased synthesis of glutamine. Alpha ketoglutarate is consumed and not available to reach other processes such as TCA ...
Microbial alteration of stable nitrogen and carbon isotopic
... acid, deamination and utilization of an excess ammonia pool. No change in fractionation, however, was observed with the uptake of an increasing percentage of the substrate (up to 97.7% of the alanine); therefore, fractionation during uptake is minimal. To test isotopic fractionations during deaminat ...
... acid, deamination and utilization of an excess ammonia pool. No change in fractionation, however, was observed with the uptake of an increasing percentage of the substrate (up to 97.7% of the alanine); therefore, fractionation during uptake is minimal. To test isotopic fractionations during deaminat ...
Schuenemann_Cytochrome P450
... substrate-free cytochrome P450cam and its Y96F and Y96F-Y75F mutants with peroxy acids has been performed [11]. High-field EPR studies at 285 and 94 GHz on freeze-quenched wild type and Y96F samples reveal g-tensor components for the radical (stretched gx-values from 2.0078-2.0064, gy = 2.0043, and ...
... substrate-free cytochrome P450cam and its Y96F and Y96F-Y75F mutants with peroxy acids has been performed [11]. High-field EPR studies at 285 and 94 GHz on freeze-quenched wild type and Y96F samples reveal g-tensor components for the radical (stretched gx-values from 2.0078-2.0064, gy = 2.0043, and ...
Lecture 6
... substrate and inhibitor compete for binding to the same active site or noncompetitively, when the inhibitor binds somewhere else on the enzyme molecule reducing its efficiency. • The distinction can be determined by plotting enzyme activity with and without the inhibitor present. • Competitive Inhib ...
... substrate and inhibitor compete for binding to the same active site or noncompetitively, when the inhibitor binds somewhere else on the enzyme molecule reducing its efficiency. • The distinction can be determined by plotting enzyme activity with and without the inhibitor present. • Competitive Inhib ...
Slide 1
... • Once again, we see selectivity in peptide bond formation – As in the ribosome, the NRPS can orient the reacting centres in close proximity to eachother, while physically blocking other sites ...
... • Once again, we see selectivity in peptide bond formation – As in the ribosome, the NRPS can orient the reacting centres in close proximity to eachother, while physically blocking other sites ...
Catalytic triad

A catalytic triad refers to the three amino acid residues that function together at the centre of the active site of some hydrolase and transferase enzymes (e.g. proteases, amidases, esterases, acylases, lipases and β-lactamases). An Acid-Base-Nucleophile triad is a common motif for generating a nucleophilic residue for covalent catalysis. The residues form a charge-relay network to polarise and activate the nucleophile, which attacks the substrate, forming a covalent intermediate which is then hydrolysed to regenerate free enzyme. The nucleophile is most commonly a serine or cysteine amino acid, but occasionally threonine. Because enzymes fold into complex three-dimensional structures, the residues of a catalytic triad can be far from each other along the amino-acid sequence (primary structure), however, they are brought close together in the final fold.As well as divergent evolution of function (and even the triad's nucleophile), catalytic triads show some of the best examples of convergent evolution. Chemical constraints on catalysis have led to the same catalytic solution independently evolving in at least 23 separate superfamilies. Their mechanism of action is consequently one of the best studied in biochemistry.