Laws
... • During a chemical reaction, a group combines 5.00 grams of sodium and 7.72 grams of chlorine. The result of the reaction was 12.72 grams of sodium chloride. Which law does this support? ...
... • During a chemical reaction, a group combines 5.00 grams of sodium and 7.72 grams of chlorine. The result of the reaction was 12.72 grams of sodium chloride. Which law does this support? ...
Topic 2 Microscopic World I
... Both statements are true and the 2nd statement is a correct explanation of the 1st statement. Both statements are true but the 2nd statement is NOT a correct explanation of the 1st statement. The 1st statement is false but the 2nd statement is true. Both statements are false. ...
... Both statements are true and the 2nd statement is a correct explanation of the 1st statement. Both statements are true but the 2nd statement is NOT a correct explanation of the 1st statement. The 1st statement is false but the 2nd statement is true. Both statements are false. ...
Nitrogen Cycle in Aquaponics
... important to aquaponics? When the nitrite levels reach a certain point, nitriteoxidizing bacteria (nitrospira) colonize the system and convert the nitrites to nitrates.....which become plant food. The plants take up the nitrates (and other compounds) and as they grow and are harvested, nitrogen ...
... important to aquaponics? When the nitrite levels reach a certain point, nitriteoxidizing bacteria (nitrospira) colonize the system and convert the nitrites to nitrates.....which become plant food. The plants take up the nitrates (and other compounds) and as they grow and are harvested, nitrogen ...
Ch#4 Atoms and Elements
... Relative Atomic Mass of Hydrogen When computing the average atomic mass of any element the radioactive (unstable) isotopes are excluded since there relative abundances are slowly decreasing. Hydrogen has three isotopes. The first two protium and deuterium are stable isotopes and the third tritium i ...
... Relative Atomic Mass of Hydrogen When computing the average atomic mass of any element the radioactive (unstable) isotopes are excluded since there relative abundances are slowly decreasing. Hydrogen has three isotopes. The first two protium and deuterium are stable isotopes and the third tritium i ...
Unit 3 Study Guide: Atomic Structure and Nuclear
... _______________ 2. Philosophers formulated explanations about the nature of matter based on their own experiences. _______________ 3. Both Democritus and Dalton suggested that matter is made up of atoms. _______________ 4. Dalton's atomic theory stated that atoms separate, combine, or rearrange in c ...
... _______________ 2. Philosophers formulated explanations about the nature of matter based on their own experiences. _______________ 3. Both Democritus and Dalton suggested that matter is made up of atoms. _______________ 4. Dalton's atomic theory stated that atoms separate, combine, or rearrange in c ...
ISOTOPIC NOTATION isotopes are atoms with the same number of
... An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. ...
... An instrument called the mass spectrometer is generally used to determine the percentages and individual masses of each isotope. ...
Ch#4 Atoms and Elements
... and deuterium are stable isotopes and the third tritium is unstable, thus excluded in the calculation. The relative abundance of isotopes of a particular element is constant here on our planet Earth. Mass spectroscopy gives information of relative abundances and relative masses of isotopes. ...
... and deuterium are stable isotopes and the third tritium is unstable, thus excluded in the calculation. The relative abundance of isotopes of a particular element is constant here on our planet Earth. Mass spectroscopy gives information of relative abundances and relative masses of isotopes. ...
Atomic Structure PPQs 2
... (iii) Calculate the volume of carbon dioxide, in dm3, that would react with 4.56 g of potassium superoxide. Assume that 1.00 mol of a gas occupies 24 dm3 under the conditions of the experiment. ...
... (iii) Calculate the volume of carbon dioxide, in dm3, that would react with 4.56 g of potassium superoxide. Assume that 1.00 mol of a gas occupies 24 dm3 under the conditions of the experiment. ...
Build An Atom - ChemConnections
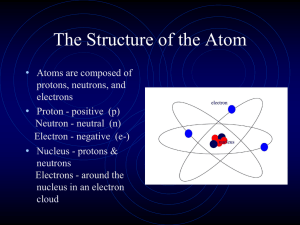
... a. The identity of an element and its position in the periodic table. b. Whether an atom is neutral or an ion (cation or anion) and its respective charge. c. Orbits versus clouds. d. The total mass ...
... a. The identity of an element and its position in the periodic table. b. Whether an atom is neutral or an ion (cation or anion) and its respective charge. c. Orbits versus clouds. d. The total mass ...
Chemical Element
... nucleosynthesis and Supernova nucleosynthesis. However intergalactic space can still closely resemble the primordial abundance, unless it has been enriched by some means. The following table shows the ten most common elements in our galaxy (estimated spectroscopically), as measured in parts per mill ...
... nucleosynthesis and Supernova nucleosynthesis. However intergalactic space can still closely resemble the primordial abundance, unless it has been enriched by some means. The following table shows the ten most common elements in our galaxy (estimated spectroscopically), as measured in parts per mill ...
File
... 34.96885 amu and percent abundance of 75.77% 36.96590 amu and percent abundance of 24.23% a. What is the average atomic mass for this element? b. What is the identity of the element? 5. Two isotopes of potassium exist in nature: potassium-39 and potassium-41. Potassium has an atomic mass of 39.0983 ...
... 34.96885 amu and percent abundance of 75.77% 36.96590 amu and percent abundance of 24.23% a. What is the average atomic mass for this element? b. What is the identity of the element? 5. Two isotopes of potassium exist in nature: potassium-39 and potassium-41. Potassium has an atomic mass of 39.0983 ...
Elements and Compounds
... • Nature contains 19.9% 10B and 80.1% 11B. What is the weighted average? • 10B: 0.199 x 10.013 AMU = 1.99 AMU • 11B: 0.801 x 11.009 AMU = 8.82 AMU ...
... • Nature contains 19.9% 10B and 80.1% 11B. What is the weighted average? • 10B: 0.199 x 10.013 AMU = 1.99 AMU • 11B: 0.801 x 11.009 AMU = 8.82 AMU ...
vibrations and waves
... ____________________ 3. Both Democritus and Dalton suggested that matter is made up of atoms. ____________________ 4. Dalton’s atomic theory stated that atoms separate, combine, or rearrange in chemical reactions. ____________________ 5. Dalton’s atomic theory stated that matter is mostly empty spac ...
... ____________________ 3. Both Democritus and Dalton suggested that matter is made up of atoms. ____________________ 4. Dalton’s atomic theory stated that atoms separate, combine, or rearrange in chemical reactions. ____________________ 5. Dalton’s atomic theory stated that matter is mostly empty spac ...
PowerPoint
... After completing this lesson, you will be able to: • Identify the structural differences between the isotopes of elements. • Determine the weighted average mass of an element when given the appropriate data. ...
... After completing this lesson, you will be able to: • Identify the structural differences between the isotopes of elements. • Determine the weighted average mass of an element when given the appropriate data. ...
Heats of Formation WS
... Heats of Formation 1. For each of the following compounds, write a balanced thermochemical equation depicting the formation of one mole of the compound from its elements in their standard states and use the appendix to obtain the value of ∆Hfº. [a] NO2 (g) ...
... Heats of Formation 1. For each of the following compounds, write a balanced thermochemical equation depicting the formation of one mole of the compound from its elements in their standard states and use the appendix to obtain the value of ∆Hfº. [a] NO2 (g) ...
Chem-130 Test Lecture
... Isotopes, Atomic Numbers and Mass Numbers Atomic number is the number of protons in the nucleus. All atoms of the same element have the same atomic number. Mass number is the sum of the number of protons & neutrons The number of neutrons in the nucleus is given by the mass number minus the atomi ...
... Isotopes, Atomic Numbers and Mass Numbers Atomic number is the number of protons in the nucleus. All atoms of the same element have the same atomic number. Mass number is the sum of the number of protons & neutrons The number of neutrons in the nucleus is given by the mass number minus the atomi ...
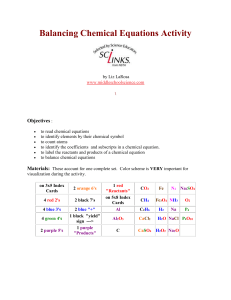
Balancing Chemical Equations Activity by Liz LaRosa www
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
... The index cards are a bit time consuming to create. I had some students help at lunch time for a few days. Once done, you can laminate them and have them forever! The materials account for one complete set which is good for 2-3 students to use. Print activity cards on card stock instead of making in ...
Pennium (gr. 9-12)
... foundational understanding of chemistry. The number of protons is given in the periodic table (which is the atomic number). The number of electrons can be figured out by subtracting the charge of the given element by the number of protons. An example is P3-: by looking on the periodic table of eleme ...
... foundational understanding of chemistry. The number of protons is given in the periodic table (which is the atomic number). The number of electrons can be figured out by subtracting the charge of the given element by the number of protons. An example is P3-: by looking on the periodic table of eleme ...
Unit 2
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
Unit 2
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
... assignment focuses on chapters 1,2,3 and chapter 10 up to, and including, section 10.6. You must also spend some time memorizing the common ion chart at the end of this packet. Also at the end of the assignment is a sheet of elements. You do not have to turn this sheet in, but you must learn the sym ...
Nuts,Bolts and Isotopes- Average Atomic Mass Activity
... exactly the same. Not all carbon atoms are alike! There are three different types of carbon atoms (Carbon 12, 13 and14). Each type of carbon atom has a different mass. We refer to these atoms which are the same, but different, as isotopes. Isotopes are atoms of the same type, but with differing numb ...
... exactly the same. Not all carbon atoms are alike! There are three different types of carbon atoms (Carbon 12, 13 and14). Each type of carbon atom has a different mass. We refer to these atoms which are the same, but different, as isotopes. Isotopes are atoms of the same type, but with differing numb ...
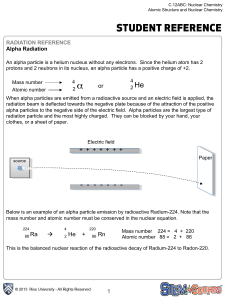
2 α
... days. Most radioactive isotopes that are used in nuclear medicine are selected because they do not last very long. Their half-life, or the amount of time it takes for ½ of the amount of the isotope to decay is short. The radioactive isotope soon forms a more stable element that is not harmful to bod ...
... days. Most radioactive isotopes that are used in nuclear medicine are selected because they do not last very long. Their half-life, or the amount of time it takes for ½ of the amount of the isotope to decay is short. The radioactive isotope soon forms a more stable element that is not harmful to bod ...
U4 Project
... isotopes for your element. Disregard the numbers in the parentheses behind the percent and the exact mass. of each isotope. Write down the exact mass and percent abundance for each isotope in the format seen below. Reminder: Your element may have more than 2 isotopes and you must change each percent ...
... isotopes for your element. Disregard the numbers in the parentheses behind the percent and the exact mass. of each isotope. Write down the exact mass and percent abundance for each isotope in the format seen below. Reminder: Your element may have more than 2 isotopes and you must change each percent ...
average atomic mass
... The average atomic mass of an element depends of both the mass and relative abundance of each of the element’s isotopes. Most elements occur in nature as a specific mixture of isotopes. They don’t occur equally… some are more abundant than others. ...
... The average atomic mass of an element depends of both the mass and relative abundance of each of the element’s isotopes. Most elements occur in nature as a specific mixture of isotopes. They don’t occur equally… some are more abundant than others. ...
Document
... A) nuclear power plants. B) medical X rays. C) the natural environment. D) fallout from past and present testing of nuclear weapons. 2) In order for an atom to decay to an element which is one greater in atomic number, it can emit A) one beta particle. B) one positron and 2 beta particles. C) one al ...
... A) nuclear power plants. B) medical X rays. C) the natural environment. D) fallout from past and present testing of nuclear weapons. 2) In order for an atom to decay to an element which is one greater in atomic number, it can emit A) one beta particle. B) one positron and 2 beta particles. C) one al ...
Isotope analysis

Isotope analysis is the identification of isotopic signature, the distribution of certain stable isotopes and chemical elements within chemical compounds. This can be applied to a food web to make it possible to draw direct inferences regarding diet, trophic level, and subsistence. Variations in isotope ratios from isotopic fractionation are measured using mass spectrometry, which separates the different isotopes of an element on the basis of their mass-to-charge ratio.The ratios of isotopic oxygen are also differentially affected by global weather patterns and regional topography as moisture is transported. Areas of lower humidity cause the preferential loss of 18O water in the form of vapor and precipitation. Furthermore, evaporated 16O water returns preferentially to the atmospheric system as it evaporates and 18O remains in liquid form or is incorporated into the body water of plants and animals.