Chapter 6 HEAT CAPACITY, ENTHALPY, ENTROPY, AND
... As a result of experimental measurement, Dulong and Petit introduced an empirical rule in 1819 which states that the molar heat capacities of all solid elements have the value 3R(=24.9 J/K), and, in 1865, Kopp introduced a rule which states that, at ordinary temperatures, the molar heat capacity of ...
... As a result of experimental measurement, Dulong and Petit introduced an empirical rule in 1819 which states that the molar heat capacities of all solid elements have the value 3R(=24.9 J/K), and, in 1865, Kopp introduced a rule which states that, at ordinary temperatures, the molar heat capacity of ...
2.26 MB - KFUPM Resources v3
... at higher sat. temperature. Note that the sat. liquid specific volume (vsat,l ) will increase while the sat. vapor specific volume ...
... at higher sat. temperature. Note that the sat. liquid specific volume (vsat,l ) will increase while the sat. vapor specific volume ...
Answer ALL questions in section A and Any Three questions in
... Name the four simple types of processes used in thermodynamic studies and for each type state the variable held constant. [8 marks] ...
... Name the four simple types of processes used in thermodynamic studies and for each type state the variable held constant. [8 marks] ...
Thermal Transport Measurements of Individual Multiwalled Nanotubes
... we can estimate the T-independent lst ⬃ 500 nm for the MWNT. Note that this value is an order of magnitude higher than previous estimations from bulk measurements [7] and is comparable to the length of the measured MWNT (2.5 mm). Thus below room temperature where the phonon-phonon umklapp scattering ...
... we can estimate the T-independent lst ⬃ 500 nm for the MWNT. Note that this value is an order of magnitude higher than previous estimations from bulk measurements [7] and is comparable to the length of the measured MWNT (2.5 mm). Thus below room temperature where the phonon-phonon umklapp scattering ...
III. PCB thermophysical properties assessment
... influence of materials THP not only in assembling area but also in the development, specification, and quality control of materials used in electronics packaging and thermal management. The THP are measured for pure substances and intrinsic materials. In the electronic packaging the necessity of THP ...
... influence of materials THP not only in assembling area but also in the development, specification, and quality control of materials used in electronics packaging and thermal management. The THP are measured for pure substances and intrinsic materials. In the electronic packaging the necessity of THP ...
Sahand University of Technology
... Theories of Adhesion • The six theories of adhesion: 3. Diffusion Theory, – The diffusion theory takes the view that polymers in contact may interdiffuse, so that the initial boundary is eventually removed . – Such interdiffusion will occur only if the polymer chains are mobile (i.e. the temperatur ...
... Theories of Adhesion • The six theories of adhesion: 3. Diffusion Theory, – The diffusion theory takes the view that polymers in contact may interdiffuse, so that the initial boundary is eventually removed . – Such interdiffusion will occur only if the polymer chains are mobile (i.e. the temperatur ...
Temperature Profiling of VCSELs by Thermoreflectance Microscopy
... Typical thermoreflectance images of an operating oxide-confined SM VCSEL are shown in Fig. 2(a). These images show spatially resolved changes in surface temperature of the VCSEL in response to a sinusoidal modulation of the injection current. As expected, the VCSELs are hotter near the central axis ...
... Typical thermoreflectance images of an operating oxide-confined SM VCSEL are shown in Fig. 2(a). These images show spatially resolved changes in surface temperature of the VCSEL in response to a sinusoidal modulation of the injection current. As expected, the VCSELs are hotter near the central axis ...
The magnetic properties of the high pressure
... V,, and lies along a, or the spin axis and V,, would usually expect that the internal magare in the bc plane and less than 13” apart. It netic field for 4-coordination would be sighas proved difficult to reconcile the former nificantly smaller than that for 6-coordinawith the point charge calculatio ...
... V,, and lies along a, or the spin axis and V,, would usually expect that the internal magare in the bc plane and less than 13” apart. It netic field for 4-coordination would be sighas proved difficult to reconcile the former nificantly smaller than that for 6-coordinawith the point charge calculatio ...
Module
... Figure 3(a) to (c) schematically shows the uniaxial tensile, shear and hydrostatic compression on a typical block of material. When a sample of material is stretched in one direction it tends to get thinner in the other two directions. The Poisson's ratio becomes important to highlight this characte ...
... Figure 3(a) to (c) schematically shows the uniaxial tensile, shear and hydrostatic compression on a typical block of material. When a sample of material is stretched in one direction it tends to get thinner in the other two directions. The Poisson's ratio becomes important to highlight this characte ...
Consistent Application of the Boltzmann Distribution to Residual
... measurements of its heat capacity (and phase changes) from 0 K to the temperature of the gas. Calorimetry, like any thermodynamic measurement, yields a difference, and thus gives ∆S rather than absolute entropy values. Unlike the corresponding energy or enthalpy, entropy is normally set equal to zer ...
... measurements of its heat capacity (and phase changes) from 0 K to the temperature of the gas. Calorimetry, like any thermodynamic measurement, yields a difference, and thus gives ∆S rather than absolute entropy values. Unlike the corresponding energy or enthalpy, entropy is normally set equal to zer ...
thermodynamic states
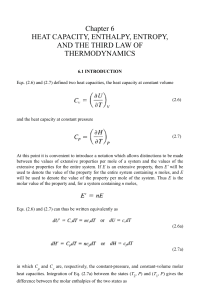
... Above integration result implies existence of new function of states, S ...
... Above integration result implies existence of new function of states, S ...
Pdf
... J ~ by highvacancies, interstitials, and Bjerrum orientational d e f e ~ t s . ~ pressure injection of liquid water into cryofluids.20 In Cubic ice (IC)has virtually the same density as hexagonal comparison with ice Ih these substances surely have relice (Ih) at the low temperatures where both are k ...
... J ~ by highvacancies, interstitials, and Bjerrum orientational d e f e ~ t s . ~ pressure injection of liquid water into cryofluids.20 In Cubic ice (IC)has virtually the same density as hexagonal comparison with ice Ih these substances surely have relice (Ih) at the low temperatures where both are k ...
How to Use Capacitive Sensors to Help Solve Difficult Level
... Since capacitive sensors are used to sense liquids, any liquid touching the actual sensor will cause it to lock on. Because capacitive sensors cannot sense through metal, applications that require liquid level detection through a metal container wall require special sight glass and tank well fitting ...
... Since capacitive sensors are used to sense liquids, any liquid touching the actual sensor will cause it to lock on. Because capacitive sensors cannot sense through metal, applications that require liquid level detection through a metal container wall require special sight glass and tank well fitting ...
Effect of carbon black content on the stress relaxation of natural rubber
... Keywords: Unfilled and Carbon Black filled Natural Rubber, Stress Relaxation, Structural changes Introduction and objectives In several applications of rubbers and rubber compounds – such as tires – product performance is largely determined by material hysteretic behaviour and strength, which are in ...
... Keywords: Unfilled and Carbon Black filled Natural Rubber, Stress Relaxation, Structural changes Introduction and objectives In several applications of rubbers and rubber compounds – such as tires – product performance is largely determined by material hysteretic behaviour and strength, which are in ...
Glass transition
The glass–liquid transition or glass transition for short is the reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass. Supercooling a viscous liquid into the glass state is called vitrification, from the Latin vitreum, ""glass"" via French vitrifier.Despite the massive change in the physical properties of a material through its glass transition, the transition is not itself a phase transition of any kind; rather it is a laboratory phenomenon extending over a range of temperature and defined by one of several conventions. Such conventions include a constant cooling rate (20 K/min) and a viscosity threshold of 1012 Pa·s, among others. Upon cooling or heating through this glass-transition range, the material also exhibits a smooth step in the thermal-expansion coefficient and in the specific heat, with the location of these effects again being dependent on the history of the material. However, the question of whether some phase transition underlies the glass transition is a matter of continuing research.The glass-transition temperature Tg is always lower than the melting temperature, Tm, of the crystalline state of the material, if one exists.