Calculating Enthalpy Changes
... The temperature dependence of DGo As we have shown previously, DG, will decrease until it reaches 0. Then we have reached equilibrium. The equilibrium condition is DGo = -RT ln K Next we consider the fact that we can use the temperature dependence of the free energy to obtain information about the ...
... The temperature dependence of DGo As we have shown previously, DG, will decrease until it reaches 0. Then we have reached equilibrium. The equilibrium condition is DGo = -RT ln K Next we consider the fact that we can use the temperature dependence of the free energy to obtain information about the ...
Chemical Hygiene Plan - Highly reactive chemicals
... • Explosives should be stored in a cool, dry, and protected area. Segregate from other material that could create a serious risk to life or property should an accident occur. Organic Peroxides are one of the most hazardous chemicals commonly used in laboratories. Most organic peroxides are sensitive ...
... • Explosives should be stored in a cool, dry, and protected area. Segregate from other material that could create a serious risk to life or property should an accident occur. Organic Peroxides are one of the most hazardous chemicals commonly used in laboratories. Most organic peroxides are sensitive ...
Fractional Distillation
... In practical terms, it is important to keep the fractionating column very hot. We can do this by wrapping the column in glass wool and by shinning a heat lamp on it. The efficiency of a fractionating column is typically reported in terms of its Hold-Up and its Number of Theoretical Plates. The Hold- ...
... In practical terms, it is important to keep the fractionating column very hot. We can do this by wrapping the column in glass wool and by shinning a heat lamp on it. The efficiency of a fractionating column is typically reported in terms of its Hold-Up and its Number of Theoretical Plates. The Hold- ...
The third law
... Heat Capacity (C), is the slope of a graph of the value of the internal energy plotted against temperature. ...
... Heat Capacity (C), is the slope of a graph of the value of the internal energy plotted against temperature. ...
Whole version
... Just like a description of Mesopotamia can begin with Adam and Eve in the Garden of Eden, or in medias res with the current events in Iraq, the calculation of chemical reactions can begin with an introduction to the quantum theory and quantum statistics, or in medias res with a definition of the che ...
... Just like a description of Mesopotamia can begin with Adam and Eve in the Garden of Eden, or in medias res with the current events in Iraq, the calculation of chemical reactions can begin with an introduction to the quantum theory and quantum statistics, or in medias res with a definition of the che ...
KEMS448 Physical Chemistry Advanced Laboratory Work
... liquid to transfer into the gas phase, striving for a greater entropy in the system. If the liquid is a solution of a solvent and a dissolved substance, less solvent molecules are able to transfer into the gas phase from the liquid (Figure 3). From this, it follows that the vapour pressure decreases ...
... liquid to transfer into the gas phase, striving for a greater entropy in the system. If the liquid is a solution of a solvent and a dissolved substance, less solvent molecules are able to transfer into the gas phase from the liquid (Figure 3). From this, it follows that the vapour pressure decreases ...
Chemistry 1
... driving in a piston, and in the process its temperature was increased from T1 to T2. What is the entropy change of the gas? Assuming that the gas behalves ideally and express your answer in terms of P1, P2, T1, T2 and R only. (10 %) ...
... driving in a piston, and in the process its temperature was increased from T1 to T2. What is the entropy change of the gas? Assuming that the gas behalves ideally and express your answer in terms of P1, P2, T1, T2 and R only. (10 %) ...
The origin and status of the Arrhenius equation
... rate constant could he correlated by one simple equation (3). I t still hears his name and is widely regarded as one of the most important equations in physical chemistry. Svante August Arrhenius, horn in 1859, was initially a student at Uppsala in Sweden. In Stockholm, he began in 1882 the series o ...
... rate constant could he correlated by one simple equation (3). I t still hears his name and is widely regarded as one of the most important equations in physical chemistry. Svante August Arrhenius, horn in 1859, was initially a student at Uppsala in Sweden. In Stockholm, he began in 1882 the series o ...
SOLUBILITY OF GASES AT 25 C AND HIGH PRESSURES: THE
... useful to correlate the activity coefficient, which refers to a hypothetical liquid solvent. The fugacities presented in figure 5 depend only on solvit properties, therefore are independent on solvent, so that it is possible to use it for calculation of activity coefficients of solvits both in polar ...
... useful to correlate the activity coefficient, which refers to a hypothetical liquid solvent. The fugacities presented in figure 5 depend only on solvit properties, therefore are independent on solvent, so that it is possible to use it for calculation of activity coefficients of solvits both in polar ...
DOC-Document
... Hz at a signal voltage of 100 mV and giga-ohm sealed impedance was obtained continuously over four days. Finally, the cantilever tip in AFM was utilized to estimate the bilayer thickness and to calculate the rupture force at the interface of the tip and the SLB. We anticipate that a silicon-based, m ...
... Hz at a signal voltage of 100 mV and giga-ohm sealed impedance was obtained continuously over four days. Finally, the cantilever tip in AFM was utilized to estimate the bilayer thickness and to calculate the rupture force at the interface of the tip and the SLB. We anticipate that a silicon-based, m ...
The Effect of Water and light Alcohols on the Viscosity of Ionic Liquids
... being manufactured, the availability of physical and chemical data about these unusual liquids at different temperatures and under different conditions is often difficult to locate. This data is needed before industrial applications can be developed. In most cases, feasibility studies are not possib ...
... being manufactured, the availability of physical and chemical data about these unusual liquids at different temperatures and under different conditions is often difficult to locate. This data is needed before industrial applications can be developed. In most cases, feasibility studies are not possib ...
AP Chemistry
... bonds absorbs energy, therefore the chemical system gains bond energy and the surroundings lose energy, typically in the form of heat. In contrast, forming bonds releases energy; resulting in lose of energy by the chemical system and a gain in energy by the surroundings (also in the form of heat). W ...
... bonds absorbs energy, therefore the chemical system gains bond energy and the surroundings lose energy, typically in the form of heat. In contrast, forming bonds releases energy; resulting in lose of energy by the chemical system and a gain in energy by the surroundings (also in the form of heat). W ...
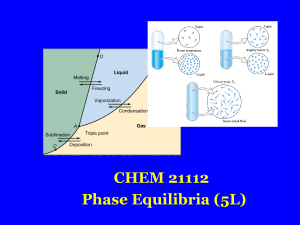
Glass transition
The glass–liquid transition or glass transition for short is the reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass. Supercooling a viscous liquid into the glass state is called vitrification, from the Latin vitreum, ""glass"" via French vitrifier.Despite the massive change in the physical properties of a material through its glass transition, the transition is not itself a phase transition of any kind; rather it is a laboratory phenomenon extending over a range of temperature and defined by one of several conventions. Such conventions include a constant cooling rate (20 K/min) and a viscosity threshold of 1012 Pa·s, among others. Upon cooling or heating through this glass-transition range, the material also exhibits a smooth step in the thermal-expansion coefficient and in the specific heat, with the location of these effects again being dependent on the history of the material. However, the question of whether some phase transition underlies the glass transition is a matter of continuing research.The glass-transition temperature Tg is always lower than the melting temperature, Tm, of the crystalline state of the material, if one exists.