
Serine Protease Mechanism
... S (TDS) and by destabilization of ES (DGd) by strain, distortion, desolvation , and similar effects. If DGb were not compensated by TDS and DGd, the formation of ES would follow the dashed line. ...
... S (TDS) and by destabilization of ES (DGd) by strain, distortion, desolvation , and similar effects. If DGb were not compensated by TDS and DGd, the formation of ES would follow the dashed line. ...
Book 2,Part 7 - GEOCITIES.ws
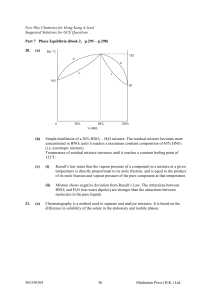
... The liquid mixture is said to be ideal if the intermolecular attraction between the molecules in the mixture is approximately equal to that in pure A and that in pure B. The liquid mixtures which approximate to ideal behaviour can be separated into their pure components by fractional distillation. T ...
... The liquid mixture is said to be ideal if the intermolecular attraction between the molecules in the mixture is approximately equal to that in pure A and that in pure B. The liquid mixtures which approximate to ideal behaviour can be separated into their pure components by fractional distillation. T ...
Space-Charge-Limited Conduction in Thin Film Al»Sb2Pb1Se7»Al
... insulator. In both the cases log I vs. v1=2 characteristics are expected to be linear in nature. In Fig. 2 the curve 'b', shows log I vs. v1=2 characteristics of a typical representative sample with lm thickness 270 nm. It is seen that the characteristic curve is still non- linear in nature, which ...
... insulator. In both the cases log I vs. v1=2 characteristics are expected to be linear in nature. In Fig. 2 the curve 'b', shows log I vs. v1=2 characteristics of a typical representative sample with lm thickness 270 nm. It is seen that the characteristic curve is still non- linear in nature, which ...
06_chapter 1
... devices. The reasons for the rising importance of thin films are many, like, tailoring the properties according to their thickness and the small mass of the material involved. The most essential parameter that determines various properties is the structure of films. Undoubtedly, modification of the ...
... devices. The reasons for the rising importance of thin films are many, like, tailoring the properties according to their thickness and the small mass of the material involved. The most essential parameter that determines various properties is the structure of films. Undoubtedly, modification of the ...
technical data - Universal Photonics
... Stacking waxes are semi-hard temporary cements used for a variety of blocking and Stacking applications. Stacking waxes are commonly used for blocking together a series of lenses or work pieces that are to be edged or profiled. Other applications include thin film blocking, Blanchard wheel work, spo ...
... Stacking waxes are semi-hard temporary cements used for a variety of blocking and Stacking applications. Stacking waxes are commonly used for blocking together a series of lenses or work pieces that are to be edged or profiled. Other applications include thin film blocking, Blanchard wheel work, spo ...
Acetic acid-water system thermodynamical correlation of vapor
... tetramer is not considered and isobaric data are directly handled : this is equivalent to neglecting the temperature effect on the activity coefficients. This is justified by the facts that the influence of both the tetramer formation and the temperature effect do not introduce serious error, on one ...
... tetramer is not considered and isobaric data are directly handled : this is equivalent to neglecting the temperature effect on the activity coefficients. This is justified by the facts that the influence of both the tetramer formation and the temperature effect do not introduce serious error, on one ...
Material
... and for irreversible processes dS > 0. In irreversible processes, the system looks for a new equilibrium state and during this process the entropy increases until it reaches its maximum value. Note that, entropy could be negative if there is heat exchange with the surroundings i.e., the system is no ...
... and for irreversible processes dS > 0. In irreversible processes, the system looks for a new equilibrium state and during this process the entropy increases until it reaches its maximum value. Note that, entropy could be negative if there is heat exchange with the surroundings i.e., the system is no ...
Lecture Slides - School of Chemical Sciences
... Why Thermodynamics? The macroscopic description of a system of ~1023 particles may involve only a few variables! “Simple systems”: Macroscopically homogeneous, isotropic, uncharged, large enough that surface effects can be neglected, not acted upon by electric, magnetic, or gravitational fields. ...
... Why Thermodynamics? The macroscopic description of a system of ~1023 particles may involve only a few variables! “Simple systems”: Macroscopically homogeneous, isotropic, uncharged, large enough that surface effects can be neglected, not acted upon by electric, magnetic, or gravitational fields. ...
Investigation of activation energy of polypropylene composite
... This work presents results of thermogravimetric analysis of composite material based on polypropylene that contains 45 wt% glass fibers. Sample decomposition took place in first major step followed by the second almost unnoticeable one. The initial decomposition temperature was established between 2 ...
... This work presents results of thermogravimetric analysis of composite material based on polypropylene that contains 45 wt% glass fibers. Sample decomposition took place in first major step followed by the second almost unnoticeable one. The initial decomposition temperature was established between 2 ...
25⁰C
... In the present study, it is shown that sonication significantly reduces the induction time for nucleation of PABA crystallized in aqueous solutions. In addition, it is shown that sonication changes the relationship between the nucleation kinetics of the α-form and the β-form in favor of the latt ...
... In the present study, it is shown that sonication significantly reduces the induction time for nucleation of PABA crystallized in aqueous solutions. In addition, it is shown that sonication changes the relationship between the nucleation kinetics of the α-form and the β-form in favor of the latt ...
Evolutionary Temperature Adaptation of Fish Sarcoplasmic Reticulum
... and small mammals. Interestingly, no such relationship was evident for membrane fluidity of sarcoplasmic reticulum (SR) membrane (Cossins, 1977). Furthermore, there was no change in the phospholipid unsaturation index of goldfish SR following acclimation to either 5, 15 or 25 °C, and only a poor cor ...
... and small mammals. Interestingly, no such relationship was evident for membrane fluidity of sarcoplasmic reticulum (SR) membrane (Cossins, 1977). Furthermore, there was no change in the phospholipid unsaturation index of goldfish SR following acclimation to either 5, 15 or 25 °C, and only a poor cor ...
Thermodynamic course year 99-00
... Two specific points in equation of state should be emphasized: A. The triple point where the solid liquid and gaseous phases coexist in equilibrium. B. The critical point where there is continuous transition between liquid and gas. At the critical point diverges to infinity. Z= ~0.3 at this point. ...
... Two specific points in equation of state should be emphasized: A. The triple point where the solid liquid and gaseous phases coexist in equilibrium. B. The critical point where there is continuous transition between liquid and gas. At the critical point diverges to infinity. Z= ~0.3 at this point. ...
Implications For Transition-State Analogs And Catalytic
... 3. AZ-28: Oxy-Cope Rearrangement Catalytic Antibody 4. Structure And Function Relationship Of AZ-28 With Transition State Analog 5. Structure And Function Relationship Of AZ-28 And Germline Antibody ...
... 3. AZ-28: Oxy-Cope Rearrangement Catalytic Antibody 4. Structure And Function Relationship Of AZ-28 With Transition State Analog 5. Structure And Function Relationship Of AZ-28 And Germline Antibody ...
Phase Polymorphism of [Mn(DMSO) ](ClO ) Studied by
... phase KIa and a single anomaly at TC2 , connected with the phase transition metastable phase KII → stable phase K0. On the DSC curve No 8 we can see one slight anomaly at TC4 , connected with the phase transition metastable phase KIII ↔ metastable phase KII, one major anomaly at TC3 , connected with ...
... phase KIa and a single anomaly at TC2 , connected with the phase transition metastable phase KII → stable phase K0. On the DSC curve No 8 we can see one slight anomaly at TC4 , connected with the phase transition metastable phase KIII ↔ metastable phase KII, one major anomaly at TC3 , connected with ...
1. INTRODUCTION
... phosphorus alkoxides have frequently been used as the phosphorus precursors for sol-gel HA synthesis, triethyl phosphate and triethyl phosphate are becoming the major materials used to prepare the precursors. The hydrolysis activity of the triethyl phosphate is relatively poor and a higher solution ...
... phosphorus alkoxides have frequently been used as the phosphorus precursors for sol-gel HA synthesis, triethyl phosphate and triethyl phosphate are becoming the major materials used to prepare the precursors. The hydrolysis activity of the triethyl phosphate is relatively poor and a higher solution ...
Glass transition
The glass–liquid transition or glass transition for short is the reversible transition in amorphous materials (or in amorphous regions within semicrystalline materials) from a hard and relatively brittle state into a molten or rubber-like state. An amorphous solid that exhibits a glass transition is called a glass. Supercooling a viscous liquid into the glass state is called vitrification, from the Latin vitreum, ""glass"" via French vitrifier.Despite the massive change in the physical properties of a material through its glass transition, the transition is not itself a phase transition of any kind; rather it is a laboratory phenomenon extending over a range of temperature and defined by one of several conventions. Such conventions include a constant cooling rate (20 K/min) and a viscosity threshold of 1012 Pa·s, among others. Upon cooling or heating through this glass-transition range, the material also exhibits a smooth step in the thermal-expansion coefficient and in the specific heat, with the location of these effects again being dependent on the history of the material. However, the question of whether some phase transition underlies the glass transition is a matter of continuing research.The glass-transition temperature Tg is always lower than the melting temperature, Tm, of the crystalline state of the material, if one exists.

















 Studied by](http://s1.studyres.com/store/data/014637611_1-0e44dc46987f4ac973f72ef81c275c20-300x300.png)

