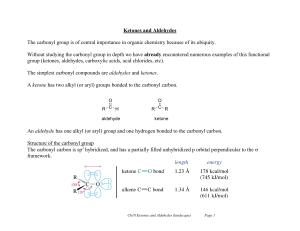
Ketones and Aldehydes
... These p orbitals form the carbon oxygen bond. The C=O double bond is like a C=C double bond except the carbonyl double bond is shorter and stronger. The carbonyl group has a large dipole moment due to the polarity of the double bond. Oxygen is more electronegative than carbon, and so the bond is p ...
... These p orbitals form the carbon oxygen bond. The C=O double bond is like a C=C double bond except the carbonyl double bond is shorter and stronger. The carbonyl group has a large dipole moment due to the polarity of the double bond. Oxygen is more electronegative than carbon, and so the bond is p ...
Chemical thermodynamics - Mahesh Tutorials Science
... The first law talks about the conservation of energy in a process but does not speak of the feasibility of a process. It does not tell whether a process will happen on its own i.e. whether the process is spontaneous or not. A spontaneous process is one which happens on its own. Example, heat always ...
... The first law talks about the conservation of energy in a process but does not speak of the feasibility of a process. It does not tell whether a process will happen on its own i.e. whether the process is spontaneous or not. A spontaneous process is one which happens on its own. Example, heat always ...
Low-Temperature Alkaline pH Hydrolysis of Oxygen-Free
... refers to the eruption of materials of low density and low melting point, which constitute part of Titan’s interior (as the plausible subsurface ocean). The presence of cryovolcanic processes has been suggested by Lorenz (1993) and Lorenz and Lunine (1996). It has been tentatively observed after Cas ...
... refers to the eruption of materials of low density and low melting point, which constitute part of Titan’s interior (as the plausible subsurface ocean). The presence of cryovolcanic processes has been suggested by Lorenz (1993) and Lorenz and Lunine (1996). It has been tentatively observed after Cas ...
Chapter 3 - Educator
... In this section we examine three simple kinds of reactions that we will see frequently throughout this chapter. Our first reason for examining these reactions is merely to become better acquainted with chemical reactions and their balanced equations. Our second reason is to consider how we might pre ...
... In this section we examine three simple kinds of reactions that we will see frequently throughout this chapter. Our first reason for examining these reactions is merely to become better acquainted with chemical reactions and their balanced equations. Our second reason is to consider how we might pre ...
Syllabus and Regulations for 2-year, 4
... Theoretical-50 Marks and Group-B: Practical- 25/30/35/40/50-Marks) each. The examiners shall forward assessment in respect of every candidate to the Principal / Controller of Examination / Coordinator P. G. Courses (as the case may be) for tabulation of the results. 3.(a) The entire course of 1000 m ...
... Theoretical-50 Marks and Group-B: Practical- 25/30/35/40/50-Marks) each. The examiners shall forward assessment in respect of every candidate to the Principal / Controller of Examination / Coordinator P. G. Courses (as the case may be) for tabulation of the results. 3.(a) The entire course of 1000 m ...
isomeria geometrica
... • Imagine looking along the Hydrogen - alpha Carbon bond of an amino acid • CORN is an acronym for -COOH; the -R group; and -NH2 • Starting at the carboxylic acid group, if you move your eyes clockwise and see the mentioned COOH group then the -R group then the -NH2 group: CORN. • L- form w3.ualg.pt ...
... • Imagine looking along the Hydrogen - alpha Carbon bond of an amino acid • CORN is an acronym for -COOH; the -R group; and -NH2 • Starting at the carboxylic acid group, if you move your eyes clockwise and see the mentioned COOH group then the -R group then the -NH2 group: CORN. • L- form w3.ualg.pt ...
Forward
... Many are made in the laboratory from alkenes, alkynes, arenes, and alcohols by reactions that you already know about and are summarized in Table 17.1. To the synthetic chemist, the most important of the reactions in Table 17.1 are the last two: the oxidation of primary alcohols to aldehydes and seco ...
... Many are made in the laboratory from alkenes, alkynes, arenes, and alcohols by reactions that you already know about and are summarized in Table 17.1. To the synthetic chemist, the most important of the reactions in Table 17.1 are the last two: the oxidation of primary alcohols to aldehydes and seco ...
Chemistry Honours - SCS Autonomous College
... Effective nuclear charge, shielding or screening effect, Slater rules, variation of effective nuclear charge in periodic table. (b) Atomic radii (van der Waals) (c) Ionic and crystal radii. (d) Covalent radii (octahedral and tetrahedral) (e) Ionization enthalpy, Successive ionization enthalpies and ...
... Effective nuclear charge, shielding or screening effect, Slater rules, variation of effective nuclear charge in periodic table. (b) Atomic radii (van der Waals) (c) Ionic and crystal radii. (d) Covalent radii (octahedral and tetrahedral) (e) Ionization enthalpy, Successive ionization enthalpies and ...
Chapter 12
... You are given moles of the reactant propane, and moles of the product carbon dioxide must be found. The balanced chemical equation must be written. Conversion from moles of C3H8 to moles of CO2 is required. The correct mole ratio has moles of unknown substance in the numerator and moles of known sub ...
... You are given moles of the reactant propane, and moles of the product carbon dioxide must be found. The balanced chemical equation must be written. Conversion from moles of C3H8 to moles of CO2 is required. The correct mole ratio has moles of unknown substance in the numerator and moles of known sub ...
Amines - ncert
... present at different positions in the parent chain, their positions are specified by giving numbers to the carbon atoms bearing –NH2 groups and suitable prefix such as di, tri, etc. is attached to the amine. The letter ‘e’ of the suffix of the hydrocarbon part is retained. For example, H2N–CH2–CH2–N ...
... present at different positions in the parent chain, their positions are specified by giving numbers to the carbon atoms bearing –NH2 groups and suitable prefix such as di, tri, etc. is attached to the amine. The letter ‘e’ of the suffix of the hydrocarbon part is retained. For example, H2N–CH2–CH2–N ...
Study Guide Chapter 4 Alcohols and Alkyl Halides
... proportions of hydrogen atom removal are given by the product of the statistical distribution and the relative rate per hydrogen. Given that a secondary hydrogen is abstracted 3.9 times faster than a primary one, we write the expression for the amount of chlorination at the primary relative to that ...
... proportions of hydrogen atom removal are given by the product of the statistical distribution and the relative rate per hydrogen. Given that a secondary hydrogen is abstracted 3.9 times faster than a primary one, we write the expression for the amount of chlorination at the primary relative to that ...
National German Competition and Problems of the IChO
... d) Calculate the mass concentration of calcium in mg/L! e) Which water supply station delivered the water? From hearsay Eileen knows that salty water is particularly healthy. She wants to raise the mass content of chloride in the pool water to 1%. 1 kg of pure salt costs €1.24. The pool has a base a ...
... d) Calculate the mass concentration of calcium in mg/L! e) Which water supply station delivered the water? From hearsay Eileen knows that salty water is particularly healthy. She wants to raise the mass content of chloride in the pool water to 1%. 1 kg of pure salt costs €1.24. The pool has a base a ...
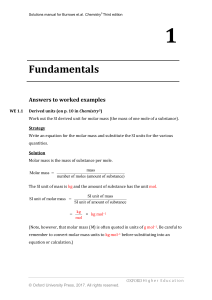
Fundamentals
... formula need to be identified. Fluorine is more electronegative, so the compound is selenium tetrafluoride. (b) Iron forms more than one type of ion, so the oxidation state needs to be indicated. The anion is perchlorate, ClO4, so the compound is iron(III) perchlorate or iron(III) chlorate(VII). Us ...
... formula need to be identified. Fluorine is more electronegative, so the compound is selenium tetrafluoride. (b) Iron forms more than one type of ion, so the oxidation state needs to be indicated. The anion is perchlorate, ClO4, so the compound is iron(III) perchlorate or iron(III) chlorate(VII). Us ...
c8h18 isomers
... ♦ Inertness to many chemicals (also called paraffins = slight affinity) C-C and C-H are strong bonds, resistant to breaking. C and H have almost the same electronegativity, therefore little polarization. ♦ No unshared electrons for acid attack ♦ Characteristic reactions: Halogenation C ...
... ♦ Inertness to many chemicals (also called paraffins = slight affinity) C-C and C-H are strong bonds, resistant to breaking. C and H have almost the same electronegativity, therefore little polarization. ♦ No unshared electrons for acid attack ♦ Characteristic reactions: Halogenation C ...
organic chemistry - Sakshieducation.com
... (ii) The oxonium salts are soluble in acid solution and regeneration of ether can be made by hydrolysis of these salts. ...
... (ii) The oxonium salts are soluble in acid solution and regeneration of ether can be made by hydrolysis of these salts. ...
Chapter 5 Principles of Chemical Reactivity: Energy and Chemical
... The third equation has water as a product, and we need to “consume” the water formed in equation two, so let’s reverse equation 3—changing the sign—AND multiply it by 6 6 H2O (g) → 6 H2 (g) + 3 O2 (g) ΔH = (+241.8)(6) kJ Adding these 3 equations gives 2 N2 (g) + 2 O2 (g) → 4 ΝΟ (g ) ...
... The third equation has water as a product, and we need to “consume” the water formed in equation two, so let’s reverse equation 3—changing the sign—AND multiply it by 6 6 H2O (g) → 6 H2 (g) + 3 O2 (g) ΔH = (+241.8)(6) kJ Adding these 3 equations gives 2 N2 (g) + 2 O2 (g) → 4 ΝΟ (g ) ...
Stoichiometry
... Chemistry - A Molecular Science (CAMS), the first half of this two-volume sequence, stressed bonding, structure, and reactivity. The material was qualitative and stressed several types of reactions and the factors that affected their relative extents of reaction. However, as the title of this text s ...
... Chemistry - A Molecular Science (CAMS), the first half of this two-volume sequence, stressed bonding, structure, and reactivity. The material was qualitative and stressed several types of reactions and the factors that affected their relative extents of reaction. However, as the title of this text s ...
Advanced Practical Organic Chemistry
... The simplest hydrocarbon is methane, CH4. This is the simplest member of a series of hydrocarbons. Each successive member of the series has one more carbon atom than the preceding member. This is shown in the table below: ...
... The simplest hydrocarbon is methane, CH4. This is the simplest member of a series of hydrocarbons. Each successive member of the series has one more carbon atom than the preceding member. This is shown in the table below: ...
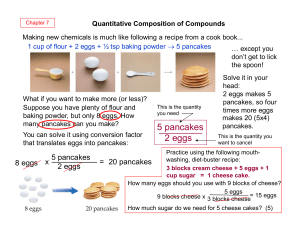
5 pancakes 2 eggs
... Molar mass: of an element is its atomic mass in grams. of a compound is the sum of the atomic masses of all atoms. Example: molar mass of NaCl is 22.99 + 35.45 = 65.44 g/mol For calculations of mole – mass – number_of particles relationship: ...
... Molar mass: of an element is its atomic mass in grams. of a compound is the sum of the atomic masses of all atoms. Example: molar mass of NaCl is 22.99 + 35.45 = 65.44 g/mol For calculations of mole – mass – number_of particles relationship: ...
Chemical Quantities and Aqueous Reactions
... outside of my office today would be below 0 °F (-17.8 °C), and even the sunniest U.S. cities would most likely be covered with snow. However, if the concentration of greenhouse gases in the atmosphere were to increase, Earth’s average temperature would rise. In recent years scientists have become co ...
... outside of my office today would be below 0 °F (-17.8 °C), and even the sunniest U.S. cities would most likely be covered with snow. However, if the concentration of greenhouse gases in the atmosphere were to increase, Earth’s average temperature would rise. In recent years scientists have become co ...
boehm_rl
... ethyl methylchloroacetate, ethyl phenylchloroacetate, and ethyl diphenylchloroacetate from the corresponding alcohols. Bissinger and Kung (32) studied the effects of varying experimental conditions upon the reactions of propyl alcohols and thionyl chloride . In order to prepare unstable propyl sulfi ...
... ethyl methylchloroacetate, ethyl phenylchloroacetate, and ethyl diphenylchloroacetate from the corresponding alcohols. Bissinger and Kung (32) studied the effects of varying experimental conditions upon the reactions of propyl alcohols and thionyl chloride . In order to prepare unstable propyl sulfi ...
Synthesis and Structural Studies of Calcium and Magnesium
... The challenge to the chemistry of the alkaline earth phosphonate target compounds includes poor solubility of compounds, and poorly understood details on the control of the metal’s coordination environment. Hence, less is known on phosphonate based alkaline earth metal organic frameworks as compared ...
... The challenge to the chemistry of the alkaline earth phosphonate target compounds includes poor solubility of compounds, and poorly understood details on the control of the metal’s coordination environment. Hence, less is known on phosphonate based alkaline earth metal organic frameworks as compared ...
Chapter 3
... the electrodes, was responsible. Furthermore, they reasoned that because cancer is the result of the uncontrolled division of abnormal cells, the compound might be useful as an anticancer drug. Platinol, the name under which cisplatin is marketed, was approved by the FDA in 1978 for the treatment of ...
... the electrodes, was responsible. Furthermore, they reasoned that because cancer is the result of the uncontrolled division of abnormal cells, the compound might be useful as an anticancer drug. Platinol, the name under which cisplatin is marketed, was approved by the FDA in 1978 for the treatment of ...
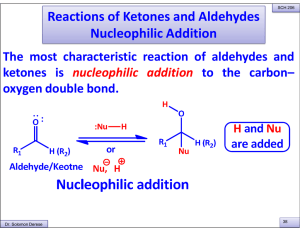
Reactions of Ketones and Aldehydes Nucleophilic Addition
... basic conditions required for anion formation nor survive in a solution containing carbanions. Acetylide ions add to carbonyl groups . The strategy that is routinely followed is to protect the carbonyl group during the reactions with which it is incompatible and then to remove the protecting group i ...
... basic conditions required for anion formation nor survive in a solution containing carbanions. Acetylide ions add to carbonyl groups . The strategy that is routinely followed is to protect the carbonyl group during the reactions with which it is incompatible and then to remove the protecting group i ...
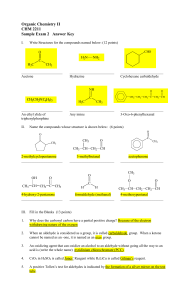
Organic Chemistry II CHM 2211 Sample Exam 2 Answer Key
... When an aldehyde is considered as a group, it is called carbaldehyde group. When a ketone cannot be named as an -one, it is named as an oxo- group. ...
... When an aldehyde is considered as a group, it is called carbaldehyde group. When a ketone cannot be named as an -one, it is named as an oxo- group. ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.