CURRICULUM SUMMARY 2016-2017 SUBJECT: Chemistry YEAR
... Oxides of nitrogen from car engines Lead compounds from leaded petrol To be able to describe and explain the presence of oxides of nitrogen in car engines and their catalytic removal. To be able to state the adverse effect of these common pollutants on buildings and on health and discuss why the ...
... Oxides of nitrogen from car engines Lead compounds from leaded petrol To be able to describe and explain the presence of oxides of nitrogen in car engines and their catalytic removal. To be able to state the adverse effect of these common pollutants on buildings and on health and discuss why the ...
Synthesis of Natural Products and Related Compounds using Enyne
... our group (Scheme 6).[22] Reactions of five- to sevenmembered cycloalkenes 14 having the substituent at the 3-position of the cycloalkene with 1c under ethylene gas afforded the cyclic compounds 15 in good yields. This reaction could proceed via the highly strained ruthenacyclobutane 16. In each cas ...
... our group (Scheme 6).[22] Reactions of five- to sevenmembered cycloalkenes 14 having the substituent at the 3-position of the cycloalkene with 1c under ethylene gas afforded the cyclic compounds 15 in good yields. This reaction could proceed via the highly strained ruthenacyclobutane 16. In each cas ...
Appendix
... 589.0 and 589.6 nm, which is the yellow range of the emission spectrum. Sodium can be vaporized at high temperatures in a sealed tube and made to give off light using two electrodes connected to a power source. Sodium vapor lighting is often used along highways and in parking lots because it provid ...
... 589.0 and 589.6 nm, which is the yellow range of the emission spectrum. Sodium can be vaporized at high temperatures in a sealed tube and made to give off light using two electrodes connected to a power source. Sodium vapor lighting is often used along highways and in parking lots because it provid ...
File - Chemistry Workshop
... A tertiary carbon is directly bonded to three other C’s. Multivalent atoms are 1º, 2º, or 3º by bonding to C’s. Univalent atom or group not really 1º, 2º, or 3º on its own - ID depends on type of carbon it is bonded to. ...
... A tertiary carbon is directly bonded to three other C’s. Multivalent atoms are 1º, 2º, or 3º by bonding to C’s. Univalent atom or group not really 1º, 2º, or 3º on its own - ID depends on type of carbon it is bonded to. ...
Amines
... Typical reagents : LiAlH4 / ether solvent, followed by aqueous work-up. Note that this reaction is different to that of other C=O compounds which reduce to alcohols (for example esters) The nature of the amine obtained depends on the substituents present on the original amide. Look at the N substitu ...
... Typical reagents : LiAlH4 / ether solvent, followed by aqueous work-up. Note that this reaction is different to that of other C=O compounds which reduce to alcohols (for example esters) The nature of the amine obtained depends on the substituents present on the original amide. Look at the N substitu ...
Document
... – Reduction of an aldehyde gives a primary alcohol (-CH2OH). – Reduction of a ketone gives a secondary alcohol (-CHOH-). ...
... – Reduction of an aldehyde gives a primary alcohol (-CH2OH). – Reduction of a ketone gives a secondary alcohol (-CHOH-). ...
Exam 1
... C. Weak dispersion forces act between propene molecules and consequently it is a gas at room temperature. D. One propene molecule will react with excess oxygen to produce three molecules of water and three molecules of carbon dioxide. Question 12 In a particular chlorination reaction, a single hydro ...
... C. Weak dispersion forces act between propene molecules and consequently it is a gas at room temperature. D. One propene molecule will react with excess oxygen to produce three molecules of water and three molecules of carbon dioxide. Question 12 In a particular chlorination reaction, a single hydro ...
Document
... Entropy Changes in a System The process of dissolving a substance can lead to either an increase or a decrease in entropy, depending on the nature of the solute. Molecular solutes (i.e. sugar): entropy increases Ionic compounds: entropy could decrease or increase ...
... Entropy Changes in a System The process of dissolving a substance can lead to either an increase or a decrease in entropy, depending on the nature of the solute. Molecular solutes (i.e. sugar): entropy increases Ionic compounds: entropy could decrease or increase ...
Functional Groups
... • Ethanol: This molecule has an OH group attached to its backbone. It is called a hydroxy functional group. Ethanol has lone pairs and polar bonds that make it reactive with a variety of reagents. The hydroxy group makes the properties of ethanol very different from the properties of ethane. ...
... • Ethanol: This molecule has an OH group attached to its backbone. It is called a hydroxy functional group. Ethanol has lone pairs and polar bonds that make it reactive with a variety of reagents. The hydroxy group makes the properties of ethanol very different from the properties of ethane. ...
Safety Quiz - WordPress.com
... Accuracy is a measurement of how close an experimental reading is to the actual answer. For example, I am 178 cm tall. If I used a ruler that said I’m 179 cm tall, that’s a reasonably accurate answer. Precision is a measurement of how repeatable an experimental reading is, and is usually denoted by ...
... Accuracy is a measurement of how close an experimental reading is to the actual answer. For example, I am 178 cm tall. If I used a ruler that said I’m 179 cm tall, that’s a reasonably accurate answer. Precision is a measurement of how repeatable an experimental reading is, and is usually denoted by ...
Chapter 10
... Given a chemical equation, or information from which it may be determined, and initial quantities of two or more reactants, (a) identify the limiting reactant, (b) calculate the theoretical yield of a specified product, assuming complete use of the limiting reactant, and (c) calculate the quantity o ...
... Given a chemical equation, or information from which it may be determined, and initial quantities of two or more reactants, (a) identify the limiting reactant, (b) calculate the theoretical yield of a specified product, assuming complete use of the limiting reactant, and (c) calculate the quantity o ...
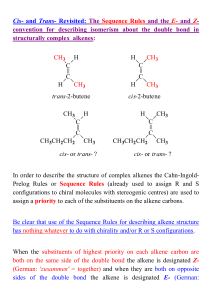
Alkenes 3 - ChemWeb (UCC)
... X = Cl, Br, I, OTs (tosylate) but X ° F B = OH-, OR- (alkoxide) etc. ...
... X = Cl, Br, I, OTs (tosylate) but X ° F B = OH-, OR- (alkoxide) etc. ...
Isoindolone Formation via Intramolecular Diels
... stage without the need for purification. Reductive amination with 4-trifluoromethoxybenzylamine (4) and a NaBH4/EtOH mixture worked well and, satisfyingly, gave an excellent purification procedure, the inseparable input mixture of 2formyl-3-carboxylate and 5-formyl-3-carboxylate isomers, after reductiv ...
... stage without the need for purification. Reductive amination with 4-trifluoromethoxybenzylamine (4) and a NaBH4/EtOH mixture worked well and, satisfyingly, gave an excellent purification procedure, the inseparable input mixture of 2formyl-3-carboxylate and 5-formyl-3-carboxylate isomers, after reductiv ...
Oxidation involving CO System ( O
... Metabolizes 1° and 2° alcohols and aldehydes containing at least one “H” attached to a-C; 1° alcohols typically go to the aldehyde then acid; 2° alcohols are converted to ketone, which cannot be further converted to the acid. The aldehyde is converted back to an alcohol by alcohol (keto) reductases ...
... Metabolizes 1° and 2° alcohols and aldehydes containing at least one “H” attached to a-C; 1° alcohols typically go to the aldehyde then acid; 2° alcohols are converted to ketone, which cannot be further converted to the acid. The aldehyde is converted back to an alcohol by alcohol (keto) reductases ...
McMurray-Fay Chapter 22 Presentation Slides
... compounds. • Carbon is tetravalent. It has four outer-shell electrons (1s22s22p2) and forms four bonds. ...
... compounds. • Carbon is tetravalent. It has four outer-shell electrons (1s22s22p2) and forms four bonds. ...
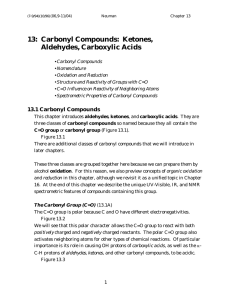
13: Carbonyl Compounds: Ketones, Aldehydes, Carboxylic Acids
... The general structure of an aldehyde looks like that of a ketone. However, aldehydes must have at least one H bonded to the C=O (Figure 13.8). Figure 13.8 As a result, the C=O group of aldehydes is always at the end of an alkane chain. In contrast, the C=O group of a ketone can never be at the end o ...
... The general structure of an aldehyde looks like that of a ketone. However, aldehydes must have at least one H bonded to the C=O (Figure 13.8). Figure 13.8 As a result, the C=O group of aldehydes is always at the end of an alkane chain. In contrast, the C=O group of a ketone can never be at the end o ...
Grignard Reagents brochure
... The reaction of Grignard reagents with carbon dioxide leads to carboxylic acids in moderate to good yields and is one of the most common methods for the synthesis of organic acids. The addition of Grignard reagents to other double bonds like C=S (i.e. in thioesters) and C=N (i.e. nitriles, imines or ...
... The reaction of Grignard reagents with carbon dioxide leads to carboxylic acids in moderate to good yields and is one of the most common methods for the synthesis of organic acids. The addition of Grignard reagents to other double bonds like C=S (i.e. in thioesters) and C=N (i.e. nitriles, imines or ...
5. Coenzyme HAD+ is derived
... steroids and their derivatives. Response to fat. Opening reaction of unsaturated higher fatty acids Boundary control №2 ...
... steroids and their derivatives. Response to fat. Opening reaction of unsaturated higher fatty acids Boundary control №2 ...
www.xtremepapers.net
... The syllabus has been constructed with a compulsory Advanced Subsidiary core. That part of the core syllabus which will be examined only in the full Advanced Level qualification is indicated in bold type. A full Advanced Level qualification requires the study of further core material together with s ...
... The syllabus has been constructed with a compulsory Advanced Subsidiary core. That part of the core syllabus which will be examined only in the full Advanced Level qualification is indicated in bold type. A full Advanced Level qualification requires the study of further core material together with s ...
BARIUM NITRATE
... The solution is highly alkaline. When the aqueous solution is cooled, crystals of barium hydroxide appear first. The aqueous solution of barium sulfide oxidizes slowly in the air forming elemental sulfur and various anions of sulfur including sulfite, thiosulfate, polysulfides and sulfate. The yello ...
... The solution is highly alkaline. When the aqueous solution is cooled, crystals of barium hydroxide appear first. The aqueous solution of barium sulfide oxidizes slowly in the air forming elemental sulfur and various anions of sulfur including sulfite, thiosulfate, polysulfides and sulfate. The yello ...
幻灯片 1
... water type, as in alcohols, ROH, or of the radical type, as in ethers which would be given the formula RO. But Williamson, by his ether synthesis, showed that mixed ethers, with two different alkyl groups, could be prepared. Ethers thus has to have the water-type formula ROR', and oxygen had the equ ...
... water type, as in alcohols, ROH, or of the radical type, as in ethers which would be given the formula RO. But Williamson, by his ether synthesis, showed that mixed ethers, with two different alkyl groups, could be prepared. Ethers thus has to have the water-type formula ROR', and oxygen had the equ ...
File
... Answers to the chapter summary worksheets (see the textbook CD-ROM) are given at the end of each chapter of this Teacher Guide. The answers to the practice unit tests are model answers that include all that examiners look for when awarding marks, together with some extra explanation. Each marking po ...
... Answers to the chapter summary worksheets (see the textbook CD-ROM) are given at the end of each chapter of this Teacher Guide. The answers to the practice unit tests are model answers that include all that examiners look for when awarding marks, together with some extra explanation. Each marking po ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.