PDF aldehydes and ketones
... conditions give this reaction where one molecule gets reduced and the other is oxidised. Thus, transfer of hydride from one molecule to the other takes place. H ...
... conditions give this reaction where one molecule gets reduced and the other is oxidised. Thus, transfer of hydride from one molecule to the other takes place. H ...
Bromine
... Isotopes Main article: Isotopes of bromine Bromine has two stable isotopes, Br (50.69 %) and Br (49.31%). At least 23 otherradioisotopes are known. Many of the bromine isotopes are fission products. Several of the heavier bromine isotopes from fission are delayed neutron emitters. All of the radioac ...
... Isotopes Main article: Isotopes of bromine Bromine has two stable isotopes, Br (50.69 %) and Br (49.31%). At least 23 otherradioisotopes are known. Many of the bromine isotopes are fission products. Several of the heavier bromine isotopes from fission are delayed neutron emitters. All of the radioac ...
10_OrganicChemistryRC
... Bring watch glasses to the fume hood as well as your box of compounds to be tested. Your instructor will place 5 drops of each compound to be tested on separate watch glasses, then carefully ignite each sample with a wooden splint. You will observe the flame and color of the smoke for each of the sa ...
... Bring watch glasses to the fume hood as well as your box of compounds to be tested. Your instructor will place 5 drops of each compound to be tested on separate watch glasses, then carefully ignite each sample with a wooden splint. You will observe the flame and color of the smoke for each of the sa ...
Topic 3 MOLE Avodagro`s number = 6.02 x 1023 things = 1 mole 1
... Calculate the mass of Ba3(PO4)2(s) formed. .600 mole Ba(NO3)2 (1 Ba3(PO4)2mole /3 mol Ba(NO3)2) = 0.200 mole Ba3(PO4)2 0.200 mole Ba3(PO4)2 (601.93g/mol) = 120 grams 11. Write the balanced equation for the complete combustion of octane, C8H18. 2C8H18 + 25O2 16CO2 + 18H2O a) How many grams of O2 are ...
... Calculate the mass of Ba3(PO4)2(s) formed. .600 mole Ba(NO3)2 (1 Ba3(PO4)2mole /3 mol Ba(NO3)2) = 0.200 mole Ba3(PO4)2 0.200 mole Ba3(PO4)2 (601.93g/mol) = 120 grams 11. Write the balanced equation for the complete combustion of octane, C8H18. 2C8H18 + 25O2 16CO2 + 18H2O a) How many grams of O2 are ...
Carbonyls
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
... Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. ...
Basic definitions for organic chemistry
... The percentage composition by mass is found by dividing the mass of an element present by the mass of the compound present, then multiplying by 100. Elemental mass of C and H can be found by allowing the substance to undergo complete combustion. From this one can find... • mass of carbon • mass of h ...
... The percentage composition by mass is found by dividing the mass of an element present by the mass of the compound present, then multiplying by 100. Elemental mass of C and H can be found by allowing the substance to undergo complete combustion. From this one can find... • mass of carbon • mass of h ...
Chemistry, 2011
... There are some very specific rules about assessments. Please refer to your NCEA handout, particularly about the ownership of work and plagiarism. Stationary and Equipment You must purchase a Student Theory Workbook ($25). You will be invoiced by the bursar upon receipt of the workbook. It is recomme ...
... There are some very specific rules about assessments. Please refer to your NCEA handout, particularly about the ownership of work and plagiarism. Stationary and Equipment You must purchase a Student Theory Workbook ($25). You will be invoiced by the bursar upon receipt of the workbook. It is recomme ...
Chemistry
... Differences between atoms give elements their different chemical properties. Atoms of one or more substances (reactants) undergo some ‘rearrangements’ during a chemical change (reaction). These rearrangements form new and different substances (products). After the chemical reaction, all the atoms of ...
... Differences between atoms give elements their different chemical properties. Atoms of one or more substances (reactants) undergo some ‘rearrangements’ during a chemical change (reaction). These rearrangements form new and different substances (products). After the chemical reaction, all the atoms of ...
CHAPTER 20 CARBOXYLIC ACIDS Organic Chemistry
... 1- Oxidation of arenes Oxidation of a substituted alkylbenzene with KMnO4 or Na2Cr2O7 or H2Cr2O7 gives a substituted benzoic acid (see Section 16.10) 1° and 2° alkyl groups can be oxidized, but tertiary groups are not ( the alkylbenzene must contain at least one benzylic hydrogen atom. Therefore, ...
... 1- Oxidation of arenes Oxidation of a substituted alkylbenzene with KMnO4 or Na2Cr2O7 or H2Cr2O7 gives a substituted benzoic acid (see Section 16.10) 1° and 2° alkyl groups can be oxidized, but tertiary groups are not ( the alkylbenzene must contain at least one benzylic hydrogen atom. Therefore, ...
2 The Nature of Matter
... interactions between different forms of matter. They include a chemical’s stability, its reactivity with other chemicals, its toxicity, and its flammability. Most physical properties describe relationships or interactions between matter and energy. A material’s electrical properties, magnetic proper ...
... interactions between different forms of matter. They include a chemical’s stability, its reactivity with other chemicals, its toxicity, and its flammability. Most physical properties describe relationships or interactions between matter and energy. A material’s electrical properties, magnetic proper ...
18.10 CONJUGATE ADDITIONS
... adds in the first step. This reaction does not occur unless there is a group attached to the double bond that can help stabilize, by resonance, the carbanion intermediate. In many cases this is the carbonyl group of an aldehyde or a ketone. However, other groups, such as the carbonyl group of an est ...
... adds in the first step. This reaction does not occur unless there is a group attached to the double bond that can help stabilize, by resonance, the carbanion intermediate. In many cases this is the carbonyl group of an aldehyde or a ketone. However, other groups, such as the carbonyl group of an est ...
St. Xavier`s College – Autonomous Mumbai Syllabus for 3 Semester
... 3. To study the mechanism of aromatic electrophilic substitution and the effect of substituents on the orientation of an incoming electrophile. 4. To familiarize the students with preparation, reactions and applications of aromatic hydrocarbons, haloarenes, phenols, ethers and epoxides and understan ...
... 3. To study the mechanism of aromatic electrophilic substitution and the effect of substituents on the orientation of an incoming electrophile. 4. To familiarize the students with preparation, reactions and applications of aromatic hydrocarbons, haloarenes, phenols, ethers and epoxides and understan ...
EXPERIMENT 2 Properties of Alkanes, Alkenes, and Alcohols
... Reactivity with 3 M NaOH(aq). Use the general procedure for solubility tests to test only those compounds that did not dissolve in water. Use 3 M NaOH(aq) as the solvent. Watch carefully for any sign of reaction as discussed in the background. Reactivity with 3 M HCl(aq). Use the general procedure f ...
... Reactivity with 3 M NaOH(aq). Use the general procedure for solubility tests to test only those compounds that did not dissolve in water. Use 3 M NaOH(aq) as the solvent. Watch carefully for any sign of reaction as discussed in the background. Reactivity with 3 M HCl(aq). Use the general procedure f ...
8fd26191dcc2fe1
... The halogen derivatives containing two halogen atom in a molecule General formula CnH2n X2 e.g. Methylene Chloride CH2 Cl2 Ethylene Chloride CH2 Br – CH2 Br ...
... The halogen derivatives containing two halogen atom in a molecule General formula CnH2n X2 e.g. Methylene Chloride CH2 Cl2 Ethylene Chloride CH2 Br – CH2 Br ...
Organic Chemistry Structures of Organic Compounds
... Organic chemistry is the chemistry generally practiced by living things. For biological efficiency, need readily available atoms and light atoms. The chemistry is primarily based on C, H, N and O; also some P, Cl, Br, S (and some transition elements in enzymes) At the moment ~ 1 x 107 organic compou ...
... Organic chemistry is the chemistry generally practiced by living things. For biological efficiency, need readily available atoms and light atoms. The chemistry is primarily based on C, H, N and O; also some P, Cl, Br, S (and some transition elements in enzymes) At the moment ~ 1 x 107 organic compou ...
Ch25_outline-of-organic-nomenclature-1
... • Aromatic hydrocarbons are cyclic hydrocarbons that have some particular features. • There is a p-orbital on each atom. – The molecule is planar. ...
... • Aromatic hydrocarbons are cyclic hydrocarbons that have some particular features. • There is a p-orbital on each atom. – The molecule is planar. ...
Organic Chemistry - City University of New York
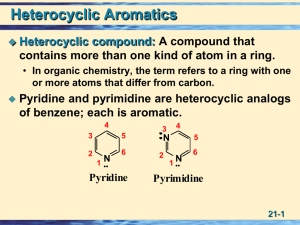
... compound: A compound that contains more than one kind of atom in a ring. • In organic chemistry, the term refers to a ring with one or more atoms that differ from carbon. ...
... compound: A compound that contains more than one kind of atom in a ring. • In organic chemistry, the term refers to a ring with one or more atoms that differ from carbon. ...
Drying Solvents: Note: When the solvent is to be distilled after
... the monohydrate is fast acting and has a high capacity (forms a heptahydrate), making it the desiccant of choice for organic extracts. It is slightly acidic so care is required with very sensitive compounds. It is mot efficient enough to be useful for pre-drying. - Molecular sieves There are sodium ...
... the monohydrate is fast acting and has a high capacity (forms a heptahydrate), making it the desiccant of choice for organic extracts. It is slightly acidic so care is required with very sensitive compounds. It is mot efficient enough to be useful for pre-drying. - Molecular sieves There are sodium ...
Chapter 20: Carboxylic Acids and Nitriles
... Why this Chapter? Amines and carbonyl compounds are the most abundant and have rich chemistry In addition to proteins and nucleic acids, a majority of pharmaceutical agents contain amine functional groups ...
... Why this Chapter? Amines and carbonyl compounds are the most abundant and have rich chemistry In addition to proteins and nucleic acids, a majority of pharmaceutical agents contain amine functional groups ...
Student Learning Outcomes (broken down by chapter…basically the
... Show how curved arrows can be used to describe the bond-breaking and bond-making steps that occur as reactants are converted into products, and how they can be used to predict the product of a reaction. Chapter 6: (2/26-3/4) Identify the orbitals used in formation of a triple bond. Name an alkyne gi ...
... Show how curved arrows can be used to describe the bond-breaking and bond-making steps that occur as reactants are converted into products, and how they can be used to predict the product of a reaction. Chapter 6: (2/26-3/4) Identify the orbitals used in formation of a triple bond. Name an alkyne gi ...
Organic Chemistry I PHS 2025 Fall 2013 Section 1 Lecture Topics
... Bond angles & hybridization of bonds connected to nitrogen, oxygen, phosphorous, & sulfur. ...
... Bond angles & hybridization of bonds connected to nitrogen, oxygen, phosphorous, & sulfur. ...
CHEM 494 Lecture 10b - UIC Department of Chemistry
... substances currently known by the names of alkalis, but alkaloids, since some of their properties they differ from alkalis considerably, and would thus find their place before the plant acids in the field of plant chemistry. ...
... substances currently known by the names of alkalis, but alkaloids, since some of their properties they differ from alkalis considerably, and would thus find their place before the plant acids in the field of plant chemistry. ...
Mole
... relationships between the amounts of reactants used and products formed by a chemical reactions; it is based on the law of conservation of mass. ...
... relationships between the amounts of reactants used and products formed by a chemical reactions; it is based on the law of conservation of mass. ...
The p-Block Elements The p-Block Elements
... pπ -p π multiple bonds with itself and with other elements having small size and high electronegativity (e.g., C, O). Heavier elements of this group do not form p π -pπ bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping. Thus, nitrogen exists as a dia ...
... pπ -p π multiple bonds with itself and with other elements having small size and high electronegativity (e.g., C, O). Heavier elements of this group do not form p π -pπ bonds as their atomic orbitals are so large and diffuse that they cannot have effective overlapping. Thus, nitrogen exists as a dia ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.