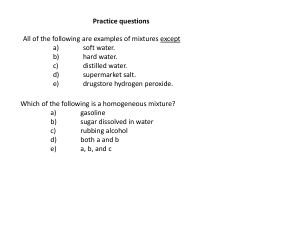
Practice questions
... a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
... a) neutrons. b) atomic number. c) nuclear charge. d) electron configuration. e) number of protons. ...
Chemical Bond - Cobb Learning
... temperature Melting and Boiling Points – Ionic compounds have much higher melting points and boiling points than covalent compounds –– Ionic compounds typically melt at several hundred degrees Celsius •They exist in a crystal state so we refer to the smallest ratio of ions in the crystal as their fo ...
... temperature Melting and Boiling Points – Ionic compounds have much higher melting points and boiling points than covalent compounds –– Ionic compounds typically melt at several hundred degrees Celsius •They exist in a crystal state so we refer to the smallest ratio of ions in the crystal as their fo ...
World of Carbon Flashcards
... on heating. They consist of polymer Thermosetting plastics do not soften or chains which have only weak forces melt on heating on account of a highly (typically van der Waals’) between cross-linked structure. them. Triglycerides are molecules formed through the condensation of one glycerol molecule ...
... on heating. They consist of polymer Thermosetting plastics do not soften or chains which have only weak forces melt on heating on account of a highly (typically van der Waals’) between cross-linked structure. them. Triglycerides are molecules formed through the condensation of one glycerol molecule ...
Organic Reactions 1
... presence of a catalyst, with the elimination of water or some other simple molecule. Catalysts commonly used in condensation reactions include acids and bases. The combination of two identical molecules is known as self-condensation. This process forms larger molecules, many of which are useful in o ...
... presence of a catalyst, with the elimination of water or some other simple molecule. Catalysts commonly used in condensation reactions include acids and bases. The combination of two identical molecules is known as self-condensation. This process forms larger molecules, many of which are useful in o ...
View PDF
... This review sheet is a list of topics and sample practice problems only. The practice problems are good representation of what to expect on the midterm, but it is not enough to just study from the review. You need to look over your notes, old review sheets, tests and quizzes, homework, etc. Ask ques ...
... This review sheet is a list of topics and sample practice problems only. The practice problems are good representation of what to expect on the midterm, but it is not enough to just study from the review. You need to look over your notes, old review sheets, tests and quizzes, homework, etc. Ask ques ...
Name
... iso- = equal (isomer: one of several organic compounds with the same molecular formula but different structures and, therefore, different properties) enanti- = opposite (enantiomer: molecules that are mirror images of each other) carb- = coal (carboxyl group: a functional group present in organic ac ...
... iso- = equal (isomer: one of several organic compounds with the same molecular formula but different structures and, therefore, different properties) enanti- = opposite (enantiomer: molecules that are mirror images of each other) carb- = coal (carboxyl group: a functional group present in organic ac ...
Ch 2-1 Properties of Matter
... 72) The wax appears to disappear because the products of the reaction—carbon dioxide and water vapor—are colorless. 79) a) yes; because the graph is a straight line, the proportion of iron to oxygen is a constant, which is true for a compound. b) no; a point for the values given wouldn’t fall on the ...
... 72) The wax appears to disappear because the products of the reaction—carbon dioxide and water vapor—are colorless. 79) a) yes; because the graph is a straight line, the proportion of iron to oxygen is a constant, which is true for a compound. b) no; a point for the values given wouldn’t fall on the ...
Preface - Wiley Online Library
... atom. Nitrogen boasts the strongest homoatomic bond rendering it chemically rather inert, but yet its fixation into plant material can be accomplished under the mildest of conditions. In his marvelous book, The Disappearing Spoon, Sean McKean eloquently summed up the idiosyncratic character of nitro ...
... atom. Nitrogen boasts the strongest homoatomic bond rendering it chemically rather inert, but yet its fixation into plant material can be accomplished under the mildest of conditions. In his marvelous book, The Disappearing Spoon, Sean McKean eloquently summed up the idiosyncratic character of nitro ...
Organic compounds
... Polymers are molecules made of many monomers. (monomer + monomer = polymer) Ex. Whole necklace ...
... Polymers are molecules made of many monomers. (monomer + monomer = polymer) Ex. Whole necklace ...
Glossary of Key Terms in Chapter Two
... oxidation (of alcohols) (12.6) the loss of electrons by a molecule, atom, or ion; in organic compounds, the gain of oxygen or loss of hydrogen; e.g., the conversion of an alcohol to an aldehyde or ketone via the use of an oxidizing agent. phenol (12.7) an organic compound that contains a hydroxyl gr ...
... oxidation (of alcohols) (12.6) the loss of electrons by a molecule, atom, or ion; in organic compounds, the gain of oxygen or loss of hydrogen; e.g., the conversion of an alcohol to an aldehyde or ketone via the use of an oxidizing agent. phenol (12.7) an organic compound that contains a hydroxyl gr ...
013368718X_CH28_437-452.indd
... The Chemistry of Carbon Organic chemistry is the study of compounds with bonds between carbon atoms. Carbon atoms have four valence electrons, allowing them to form strong covalent bonds with many other elements, including hydrogen, oxygen, phosphorus, sulfur, and nitrogen. Living organisms are made ...
... The Chemistry of Carbon Organic chemistry is the study of compounds with bonds between carbon atoms. Carbon atoms have four valence electrons, allowing them to form strong covalent bonds with many other elements, including hydrogen, oxygen, phosphorus, sulfur, and nitrogen. Living organisms are made ...
COVALENT BOND bond formed by the sharing of electrons
... Alkane chemical formulas follow a pattern The # of hydrogens =the # of carbons x 2 +2 Alkenes have at least 1 double bond ex- ethene Alkynes have at least 1 triple bond ...
... Alkane chemical formulas follow a pattern The # of hydrogens =the # of carbons x 2 +2 Alkenes have at least 1 double bond ex- ethene Alkynes have at least 1 triple bond ...
Organic Chemistry HW PSI Chemistry
... 33) Which is an acidic functional group that can dissociate and release H+ into a solution? 34) Which is a basic functional group that can accept H+ and become positively charged? The following questions refer to the molecules shown below. ...
... 33) Which is an acidic functional group that can dissociate and release H+ into a solution? 34) Which is a basic functional group that can accept H+ and become positively charged? The following questions refer to the molecules shown below. ...
Organic Chemistry HW PSI Chemistry Name
... 33) Which is an acidic functional group that can dissociate and release H+ into a solution? 34) Which is a basic functional group that can accept H+ and become positively charged? The following questions refer to the molecules shown below. ...
... 33) Which is an acidic functional group that can dissociate and release H+ into a solution? 34) Which is a basic functional group that can accept H+ and become positively charged? The following questions refer to the molecules shown below. ...
Review for SNC 2P Chemistry Unit(SPRING 2014)
... example: Elements and solutions are pure substances. (a) An atom with more electrons than protons will be a positive ion. (b) A molecular compound is held together with ionic bonds. (c) The chloride ion is an example of a polyatomic ion. (d) The chemical test for hydrogen gas of to use a glowing spl ...
... example: Elements and solutions are pure substances. (a) An atom with more electrons than protons will be a positive ion. (b) A molecular compound is held together with ionic bonds. (c) The chloride ion is an example of a polyatomic ion. (d) The chemical test for hydrogen gas of to use a glowing spl ...
unit 2 - chemistry
... covalently bonded to one O or one N H – O or H – N – may also be attracted to other O or H Weak bridges between molecules a. only 5% as strong as a covalent bond b. break and form easily c. found in H2O, proteins, and nucleic acids E. synthesis reaction – anabolic (anabolism) A + B AB (reacta ...
... covalently bonded to one O or one N H – O or H – N – may also be attracted to other O or H Weak bridges between molecules a. only 5% as strong as a covalent bond b. break and form easily c. found in H2O, proteins, and nucleic acids E. synthesis reaction – anabolic (anabolism) A + B AB (reacta ...
Key Practice Exam 3
... alkanes, ketones and carboxylic acids. Provide rationale for your ranking. Carboxylic acid > alcohol > ketones > alkanes The ranking is based on the ability of these compounds to form hydrogen bonds (their attractive intermolecular forces). Carboxylic acids can form two hydrogen bonds (they are dimm ...
... alkanes, ketones and carboxylic acids. Provide rationale for your ranking. Carboxylic acid > alcohol > ketones > alkanes The ranking is based on the ability of these compounds to form hydrogen bonds (their attractive intermolecular forces). Carboxylic acids can form two hydrogen bonds (they are dimm ...
10.4b Organic Practice Test Version 2
... a) Carbon atoms in the organic product are bonded to fewer atoms than the carbon atoms in the organic reactant. b) A hydrogen atom or functional group is replaced with a different atom or functional group. c) Atoms are added to a double or triple carbon–carbon bond. d) Two molecules are combined and ...
... a) Carbon atoms in the organic product are bonded to fewer atoms than the carbon atoms in the organic reactant. b) A hydrogen atom or functional group is replaced with a different atom or functional group. c) Atoms are added to a double or triple carbon–carbon bond. d) Two molecules are combined and ...
Elements Found in Living Things
... macromolecule. There are four classes of macromolecules (carbohydrates, lipids, proteins, and nucleic acids). Carbohydrates and lipids are made of only carbon, hydrogen, and oxygen (CHO). Proteins are made of carbon, hydrogen, oxygen, and nitrogen (CHON). Nucleic acids such as DNA and RNA contain ca ...
... macromolecule. There are four classes of macromolecules (carbohydrates, lipids, proteins, and nucleic acids). Carbohydrates and lipids are made of only carbon, hydrogen, and oxygen (CHO). Proteins are made of carbon, hydrogen, oxygen, and nitrogen (CHON). Nucleic acids such as DNA and RNA contain ca ...
lect3
... 2. The compounds can be prepared by direct reaction of metals and organic halides, by adding a metal-hydrogen bond across a C=C bond, or by transfer of organic groups between metals. 3. Grignard reagents in solution involve many complex equilibria between several organometallic species 4. Organometa ...
... 2. The compounds can be prepared by direct reaction of metals and organic halides, by adding a metal-hydrogen bond across a C=C bond, or by transfer of organic groups between metals. 3. Grignard reagents in solution involve many complex equilibria between several organometallic species 4. Organometa ...
Review Sheet: Unit 6 Name__________________ CHEMISTRY: A
... Special abbreviations are used to show the physical state of a substance in a reaction. The symbol for a liquid is ____________; for a solid, ____________; for a gas, ____________ or ____________; and for a precipitate (an ____________ solid), a ____________ or ____________. A substance that is dis ...
... Special abbreviations are used to show the physical state of a substance in a reaction. The symbol for a liquid is ____________; for a solid, ____________; for a gas, ____________ or ____________; and for a precipitate (an ____________ solid), a ____________ or ____________. A substance that is dis ...
Organosulfur compounds

Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is essential for life. Of the 20 common amino acids, two (cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin (pictured below) and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries.Sulfur shares the chalcogen group with oxygen, selenium and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium and carbon–tellurium compounds, which is true to some extent.A classical chemical test for the detection of sulfur compounds is the Carius halogen method.























