Unit 8 Student Notes
... Note the difference between solubility and the solubility product constant. Solubility, you will recall, is the amount of solute that will dissolve in a given amount of solvent. Solubility is usually expressed as the number of grams of solute per 100 grams of solvent. Assuming that there are no comp ...
... Note the difference between solubility and the solubility product constant. Solubility, you will recall, is the amount of solute that will dissolve in a given amount of solvent. Solubility is usually expressed as the number of grams of solute per 100 grams of solvent. Assuming that there are no comp ...
ΔG - Lemon Bay High School
... Plan We need to think about whether each process is consistent with our experience about the natural direction of events or whether we expect the reverse process to occur. Solve (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transfer ...
... Plan We need to think about whether each process is consistent with our experience about the natural direction of events or whether we expect the reverse process to occur. Solve (a) This process is spontaneous. Whenever two objects at different temperatures are brought into contact, heat is transfer ...
spontaneous change: entropy and free energy
... which depicts two identical glass bulbs joined by a stopcock. Initially, the bulb on the left contains an ideal gas at 1.00 atm pressure, and the bulb on the right is evacuated. When the valve is opened, the gas immediately expands into the evacuated bulb. After this expansion, the molecules are dis ...
... which depicts two identical glass bulbs joined by a stopcock. Initially, the bulb on the left contains an ideal gas at 1.00 atm pressure, and the bulb on the right is evacuated. When the valve is opened, the gas immediately expands into the evacuated bulb. After this expansion, the molecules are dis ...
AP CHEMISTRY 2005/2006
... Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ions, or molecules and the forces between them. Changes in matter involve the rearrangement and/or reorganization of atoms and/or the transfer of electrons. Rates of chemical reactions are d ...
... Chemical and physical properties of materials can be explained by the structure and the arrangement of atoms, ions, or molecules and the forces between them. Changes in matter involve the rearrangement and/or reorganization of atoms and/or the transfer of electrons. Rates of chemical reactions are d ...
2. The Thermopile
... Thermodynamics provides a means for describing the observed thermoelectric properties; however it does not provide a model which can explain the mechanisms responsible for their behavior. The required model follows from an understanding of the roles of electrons in thermoelectric behavior. The relat ...
... Thermodynamics provides a means for describing the observed thermoelectric properties; however it does not provide a model which can explain the mechanisms responsible for their behavior. The required model follows from an understanding of the roles of electrons in thermoelectric behavior. The relat ...
Chapter 5 - KFUPM Faculty List
... Energy can be converted from one form to another but can neither be created nor destroyed. EUniverse = constant First law of thermodynamics; the law of conservation of energy All forms of energy are either potential or kinetic. ...
... Energy can be converted from one form to another but can neither be created nor destroyed. EUniverse = constant First law of thermodynamics; the law of conservation of energy All forms of energy are either potential or kinetic. ...
Chemistry 12 Worksheet 2-3 Calculations Involving the
... at 448°C, Keq = 50. If 5.0 mol of HI, 0.7071 mol of H 2, and 0.7071 mol of I 2 are placed in a 1.0 L container at 448°C, will a reaction occur? (Round any answers off to 3 signif icant digits!) ...
... at 448°C, Keq = 50. If 5.0 mol of HI, 0.7071 mol of H 2, and 0.7071 mol of I 2 are placed in a 1.0 L container at 448°C, will a reaction occur? (Round any answers off to 3 signif icant digits!) ...
AP Chemistry Standards and Benchmarks
... These descriptive facts, including chemistry involved in environmental and societal issues, should not be isolated form the principles being studied but should be taught throughout the course to illustrate and illuminate the principles. The following areas should be covered: • chemical reactivity an ...
... These descriptive facts, including chemistry involved in environmental and societal issues, should not be isolated form the principles being studied but should be taught throughout the course to illustrate and illuminate the principles. The following areas should be covered: • chemical reactivity an ...
Chem 1202
... First Law of Thermodynamics (Review) Energy cannot be created or destroyed during a process; it can only be converted or transferred. Therefore ... • the total energy in the universe is constant: DEuniv = 0 = DEsys + DEsurr • any energy transferred to or from system must be transferred from or to s ...
... First Law of Thermodynamics (Review) Energy cannot be created or destroyed during a process; it can only be converted or transferred. Therefore ... • the total energy in the universe is constant: DEuniv = 0 = DEsys + DEsurr • any energy transferred to or from system must be transferred from or to s ...
Changes in Matter: Physical and Chemical Changes
... Why can't you just have instantly cooked potatoes every time? And, you may have also wondered why you needed to get the food hot in order to cook it. Well, both of these questions can be answered through chemical change concepts. ...
... Why can't you just have instantly cooked potatoes every time? And, you may have also wondered why you needed to get the food hot in order to cook it. Well, both of these questions can be answered through chemical change concepts. ...
Thermochemistry and calorimetry
... For reactions that can be initiated by combining two solutions, the temperature rise of the solution itself can provide an approximate value of the reaction enthalpy if we assume that the heat capacity of the solution is close to that of the pure water — which will be nearly true if the solutions ar ...
... For reactions that can be initiated by combining two solutions, the temperature rise of the solution itself can provide an approximate value of the reaction enthalpy if we assume that the heat capacity of the solution is close to that of the pure water — which will be nearly true if the solutions ar ...
Condensed Phase Ethanol Conversion to Higher Alcohols Tyler L
... The Peng-Robinson-Wong-Sandler (PRWS), predictive Soave-Redlich-Kwong (PSRK), and Schwartzentruber--Renon (SR-Polar) equations of state were chosen for initial model screening. These equations of state are known for accurate prediction of vapor pressures because they incorporate the acentric (ω) fac ...
... The Peng-Robinson-Wong-Sandler (PRWS), predictive Soave-Redlich-Kwong (PSRK), and Schwartzentruber--Renon (SR-Polar) equations of state were chosen for initial model screening. These equations of state are known for accurate prediction of vapor pressures because they incorporate the acentric (ω) fac ...
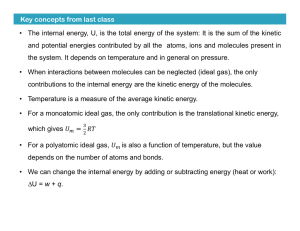
Key concepts from last class • The internal energy, U, is the total
... Heat and work cannot be stored: they are transient quantities that only apply to a system that undergoes a change in its state: There is no change in heat, because there is no initial and final heats. Instead, we’ll talk about the heat (and/or work) involved in changing the system from an initial to ...
... Heat and work cannot be stored: they are transient quantities that only apply to a system that undergoes a change in its state: There is no change in heat, because there is no initial and final heats. Instead, we’ll talk about the heat (and/or work) involved in changing the system from an initial to ...
_______1. solution a. capable of being dissolved _______2. solute
... If the temperature is decreased on the above system, which reaction increases, (forward / reverse)? There will be (more / less) SO3. 101. 2CO2 + heat 2 CO + O2 If the temperature is decreased on the above system, which reaction is increased? (forward / reverse) There will be (more / less) CO2. The ...
... If the temperature is decreased on the above system, which reaction increases, (forward / reverse)? There will be (more / less) SO3. 101. 2CO2 + heat 2 CO + O2 If the temperature is decreased on the above system, which reaction is increased? (forward / reverse) There will be (more / less) CO2. The ...