Chemical Equilibrium - The Gurukul Institute
... (NH4)2 CO3(s) ⇌ 2NH3(g) + CO2(g) + H2O(g) At a certain elevated temperature, the total pressure of the gases generated was 0.42 atm. At equilibrium. Calculate the equilibrium constant for the reaction. 10. if a given quantity of phosphorus pentachloride is heated at 250o C and allowed to come to equ ...
... (NH4)2 CO3(s) ⇌ 2NH3(g) + CO2(g) + H2O(g) At a certain elevated temperature, the total pressure of the gases generated was 0.42 atm. At equilibrium. Calculate the equilibrium constant for the reaction. 10. if a given quantity of phosphorus pentachloride is heated at 250o C and allowed to come to equ ...
Phase Rule
... sulphur, rhombic sulphur, liquid sulphur and sulphur vapour. The composition of each phase of the system can be expressed in terms of sulphur only, so, it is a one component system. (ii) Water system: It is a one component system because the composition of each of the three phases present can be exp ...
... sulphur, rhombic sulphur, liquid sulphur and sulphur vapour. The composition of each phase of the system can be expressed in terms of sulphur only, so, it is a one component system. (ii) Water system: It is a one component system because the composition of each of the three phases present can be exp ...
Physical Chemistry (SCQF level 7)
... Within this Unit candidates will need to extract and process information presented in both tabular and graphical formats developing the Core Skill of Numeracy. Candidates will gain experience in a range of calculations building competence in number. The appendix to this Unit specification contains a ...
... Within this Unit candidates will need to extract and process information presented in both tabular and graphical formats developing the Core Skill of Numeracy. Candidates will gain experience in a range of calculations building competence in number. The appendix to this Unit specification contains a ...
kauzmann temperature and the glass transition
... not only during the glass to supercooled liquid transformation (endothermic process) but also in the reverse direction (exothermic one), [6,7]. Stemming from these results the glass transition seems not to be only a kinetic phenomenon, as many authors believe, but it could be supposed that the glass ...
... not only during the glass to supercooled liquid transformation (endothermic process) but also in the reverse direction (exothermic one), [6,7]. Stemming from these results the glass transition seems not to be only a kinetic phenomenon, as many authors believe, but it could be supposed that the glass ...
PowerPoint Presentation - Chemical Equilibrium
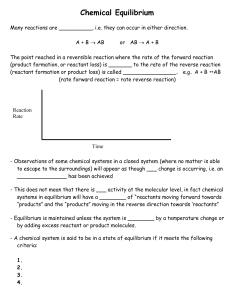
... reactions considered until now have had reactants react completely to form products. These reactions “went” only in one direction. Some reactions can react in either direction. They are “reversible”. When this occurs some amount of reactant(s) will always remain in the final reaction mixture. ...
... reactions considered until now have had reactants react completely to form products. These reactions “went” only in one direction. Some reactions can react in either direction. They are “reversible”. When this occurs some amount of reactant(s) will always remain in the final reaction mixture. ...
Magnetic relaxation in small-particle systems: ln(t/t_{0}) scaling
... type-II superconductors in which vortices are submitted to pinning by defects and dislocations, and where the application of a magnetic field creates metastable states in the vortex lattice; ' small-particle systems with a volume distribution and random orientation of easy axes which show blocking p ...
... type-II superconductors in which vortices are submitted to pinning by defects and dislocations, and where the application of a magnetic field creates metastable states in the vortex lattice; ' small-particle systems with a volume distribution and random orientation of easy axes which show blocking p ...
Determination of the enthalpy of combustion with a calorimetric
... temperature-time curve for approximately another 5 minutes. If the sample vessel is too sooty after the test, repeat the measurement, because this indicates that combustion was not complete. Fig. 2 illustrates how to determine the corrected temperature difference ∆T in order to calculate the enthalp ...
... temperature-time curve for approximately another 5 minutes. If the sample vessel is too sooty after the test, repeat the measurement, because this indicates that combustion was not complete. Fig. 2 illustrates how to determine the corrected temperature difference ∆T in order to calculate the enthalp ...
Thermodynamics Free-Response
... The reaction is endothermic (∆H = +18 kJ mol-1); an increase in temperature shifts the reaction to favor more products relative to the reactants, resulting in an increase in the value of Keq. d. Both reaction X and reaction Y have solid iodine as a reactant, but the second reactant in reaction X is ...
... The reaction is endothermic (∆H = +18 kJ mol-1); an increase in temperature shifts the reaction to favor more products relative to the reactants, resulting in an increase in the value of Keq. d. Both reaction X and reaction Y have solid iodine as a reactant, but the second reactant in reaction X is ...
General Concepts of Chemical Equilibrium
... In the reaction A+B DC+D If 0.2 mol of A is mixed with 0.5 mol of B in 1.0 L, find the equilibrium concentrations of A, B, C, and D. The equilibrium constant of the reaction is 4.0x108. It is clear that we have a high equilibrium constant which suggests that very little reactants may have been left ...
... In the reaction A+B DC+D If 0.2 mol of A is mixed with 0.5 mol of B in 1.0 L, find the equilibrium concentrations of A, B, C, and D. The equilibrium constant of the reaction is 4.0x108. It is clear that we have a high equilibrium constant which suggests that very little reactants may have been left ...
V. Diffusion
... fluctuating hydrodynamics. In this theoretical framework, diffusion is due to fluctuations whose dimensions range from the molecular scale to the macroscopic scale. Chemical diffusion increases the entropy of a system, i.e. diffusion is a spontaneous and irreversible process. Particles can spread ou ...
... fluctuating hydrodynamics. In this theoretical framework, diffusion is due to fluctuations whose dimensions range from the molecular scale to the macroscopic scale. Chemical diffusion increases the entropy of a system, i.e. diffusion is a spontaneous and irreversible process. Particles can spread ou ...
Review for Exam 3 Chem 1721/1821
... • calculation of mass or concentration of ion required to initiate precipitation Chapter 18: Thermodynamics and Equilibrium * you should review the fundamental concepts of thermochemistry that you learned in Chapter 6 * 1st Law of Thermodynamics: the energy of the universe is constant Entropy and th ...
... • calculation of mass or concentration of ion required to initiate precipitation Chapter 18: Thermodynamics and Equilibrium * you should review the fundamental concepts of thermochemistry that you learned in Chapter 6 * 1st Law of Thermodynamics: the energy of the universe is constant Entropy and th ...