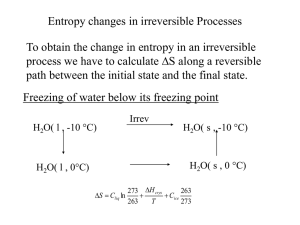
Chapter 20
... (Va / Vd ) 1 . Combining the two expressions, one clearly obtains Vb / Vc Va / Vd , or Vb / Va Vc / Vd . This result implies | Q C | / | Q H | Tc / TH and Eq. (*). The Carnot efficiency is important because it is the highest possible efficiency any engine can reach if the highest possible te ...
... (Va / Vd ) 1 . Combining the two expressions, one clearly obtains Vb / Vc Va / Vd , or Vb / Va Vc / Vd . This result implies | Q C | / | Q H | Tc / TH and Eq. (*). The Carnot efficiency is important because it is the highest possible efficiency any engine can reach if the highest possible te ...
thermodynamics properties of pure substances
... - Liquid molecular spacing is comparable to solids but their molecules can float about in groups. - There is molecular order within the groups - Weakest molecular bond strength. - Molecules in the gas phases are far apart, they have no ordered structure - The molecules move randomly and collide with ...
... - Liquid molecular spacing is comparable to solids but their molecules can float about in groups. - There is molecular order within the groups - Weakest molecular bond strength. - Molecules in the gas phases are far apart, they have no ordered structure - The molecules move randomly and collide with ...
The Third Law of Quantum Thermodynamics in the Presence of
... in elucidating such problems as why the Carnot engine can never reach 100% efficiency at finite temperatures. Although some unfulfillment may still exist the known deviations from the third law will all be cured by quantum mechanics, quantum statistics and interactions among particles according to commo ...
... in elucidating such problems as why the Carnot engine can never reach 100% efficiency at finite temperatures. Although some unfulfillment may still exist the known deviations from the third law will all be cured by quantum mechanics, quantum statistics and interactions among particles according to commo ...
1 11.8 Definition of entropy and the modern statement of the second
... initial value. To restore the initial state of the gas without causing any net change in the equilibrium states of the systems involved in this compression, we must make sure: (i) to decrease the temperature of the gas back to its original value; (ii) to move the piston back to its original position ...
... initial value. To restore the initial state of the gas without causing any net change in the equilibrium states of the systems involved in this compression, we must make sure: (i) to decrease the temperature of the gas back to its original value; (ii) to move the piston back to its original position ...
Lecture 11 - Laws of Thermodynamics
... 20-2 Heat Engines We will discuss only engines that run in a repeating cycle; the change in internal energy over a cycle is zero, as the system returns to its initial state. The high-temperature reservoir transfers an amount of heat QH to the engine, where part of it is transformed into work W and ...
... 20-2 Heat Engines We will discuss only engines that run in a repeating cycle; the change in internal energy over a cycle is zero, as the system returns to its initial state. The high-temperature reservoir transfers an amount of heat QH to the engine, where part of it is transformed into work W and ...
First Law of Thermodynamics - Derry Area School District
... likely macrostate – described by p, V, and T and obeying the ideal gas law – has so many microstates associated with it that it’s the only one you have any chance of observing. • When you allow two systems at different temperatures to exchange energy with each other, the final macrostate of the syst ...
... likely macrostate – described by p, V, and T and obeying the ideal gas law – has so many microstates associated with it that it’s the only one you have any chance of observing. • When you allow two systems at different temperatures to exchange energy with each other, the final macrostate of the syst ...
15 Thermodynamics
... Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey. ...
... Thermodynamics is the branch of physics that is built upon the fundamental laws that heat and work obey. ...
Thermo fundamentals
... 1. Easy to analyse, as System passes through series of Equilibriums. 2. Serve as Idealised Model for actual Processes to be compared for analysis. 3. Viewed as Theoretical Limit for corresponding irreversible one. Reversible Process leads to the definition of Second Law Efficiency; which is Degree o ...
... 1. Easy to analyse, as System passes through series of Equilibriums. 2. Serve as Idealised Model for actual Processes to be compared for analysis. 3. Viewed as Theoretical Limit for corresponding irreversible one. Reversible Process leads to the definition of Second Law Efficiency; which is Degree o ...
Statistical Thermodynamics -- Basic concepts.
... energy (−13.6eV ), there are 8 states with the next highest energy value (−3.4eV ), differing in their angular momentum values, and so forth. (Note here I am including spin angular momentum states in the counting.) The microstate s is then a list of all energy and angular momentum values for all the ...
... energy (−13.6eV ), there are 8 states with the next highest energy value (−3.4eV ), differing in their angular momentum values, and so forth. (Note here I am including spin angular momentum states in the counting.) The microstate s is then a list of all energy and angular momentum values for all the ...
Fundamental Concepts, Definitions and Zeroth
... Generally thermodynamics contains four laws; 1. Zeroth law: deals with thermal equilibrium and establishes a concept of temperature. 2. The First law: throws light on concept of internal energy. 3. The Second law: indicates the limit of converting heat into work and introduces the principle of incre ...
... Generally thermodynamics contains four laws; 1. Zeroth law: deals with thermal equilibrium and establishes a concept of temperature. 2. The First law: throws light on concept of internal energy. 3. The Second law: indicates the limit of converting heat into work and introduces the principle of incre ...
Chapter 12: Engineering Thermodynamics
... There are many effects whose presence during a process renders it irreversible. These include, but are not limited to, the following: heat transfer through a finite temperature difference; unrestrained expansion of a gas or liquid to a lower pressure; spontaneous chemical reaction; mixing of matter ...
... There are many effects whose presence during a process renders it irreversible. These include, but are not limited to, the following: heat transfer through a finite temperature difference; unrestrained expansion of a gas or liquid to a lower pressure; spontaneous chemical reaction; mixing of matter ...
The First Law of Thermodynamics
... The First Law of Thermodynamics A biatomic ideal gas undergoes a cycle starting at point A (2 atm, 1L). Process from A to B is an expansion at constant pressure until the volume is 2.5 L, after which is cooled at constant volume until its pressure is 1 atm. It is then compressed at constant pressur ...
... The First Law of Thermodynamics A biatomic ideal gas undergoes a cycle starting at point A (2 atm, 1L). Process from A to B is an expansion at constant pressure until the volume is 2.5 L, after which is cooled at constant volume until its pressure is 1 atm. It is then compressed at constant pressur ...
Statistical Thermodynamics and Stochastic The
... devoted to the conversion of matter and heat in such biological processes as rotting, fermentation and muscular activity. Helmholtz’s insight led him to infer a new law of nature from the complexities of his measurements on juices and extracts of meat and muscles. From experiments and brilliant gene ...
... devoted to the conversion of matter and heat in such biological processes as rotting, fermentation and muscular activity. Helmholtz’s insight led him to infer a new law of nature from the complexities of his measurements on juices and extracts of meat and muscles. From experiments and brilliant gene ...
Beverley John C. Beverley IE 500/PHI 598: Ontological Engineering
... foundations of the field of inquiry. It is with that in mind, and the lofty goals of terminological clarity, appropriate characterization of thermodynamic systems, and potential extensions into ...
... foundations of the field of inquiry. It is with that in mind, and the lofty goals of terminological clarity, appropriate characterization of thermodynamic systems, and potential extensions into ...
Chapter 15
... are four laws of thermodynamics, of which two appear on the AP Physics B exam by name (1st and 2nd). However, the concepts involved in the other two (0th and 3rd) are important to the understanding of thermodynamics as well. The zeroth law of thermodynamics states that if two systems are in equilibr ...
... are four laws of thermodynamics, of which two appear on the AP Physics B exam by name (1st and 2nd). However, the concepts involved in the other two (0th and 3rd) are important to the understanding of thermodynamics as well. The zeroth law of thermodynamics states that if two systems are in equilibr ...