Topic 2 The first law of thermodynamics
... Extensive properties: dependent on amount Intensive properties: independent on amount ...
... Extensive properties: dependent on amount Intensive properties: independent on amount ...
CHEM 240 Who am I?
... constant, its pressure my vary over a range of values. • If the pressure of one of these systems is held constant, its volume my vary over a range of values. • Thus, V and P are independent thermodynamic variables. • When one of the systems reach equilibrium at a certain P and V, all its macroscopic ...
... constant, its pressure my vary over a range of values. • If the pressure of one of these systems is held constant, its volume my vary over a range of values. • Thus, V and P are independent thermodynamic variables. • When one of the systems reach equilibrium at a certain P and V, all its macroscopic ...
Chem 521 Chemical Thermodynamics–—Syllabus, Fall 2015
... current Catalog. The attendance record is kept by roll call. Being more than 5 minutes late or missing a daily quiz is equivalent to missing a lecture. Excessive absence is defined as missing more than 10% of the lectures without excusable reasons. In addition, according to the TAMU-Commerce Procedu ...
... current Catalog. The attendance record is kept by roll call. Being more than 5 minutes late or missing a daily quiz is equivalent to missing a lecture. Excessive absence is defined as missing more than 10% of the lectures without excusable reasons. In addition, according to the TAMU-Commerce Procedu ...
system
... A physical or chemical change that occurs by itself. It does not require any outside force, and it continues until equilibrium is reached. Spontaneous processes are irreversible ...
... A physical or chemical change that occurs by itself. It does not require any outside force, and it continues until equilibrium is reached. Spontaneous processes are irreversible ...
Lecture5
... W = -Fex Δx = -p S Δx = -p ΔV Sign convention – from the point of view of the system ...
... W = -Fex Δx = -p S Δx = -p ΔV Sign convention – from the point of view of the system ...
9. Entropy 2nd and 3rd laws/ Thermodynamic processes / Droplet
... 9.1 Entropy in second and third laws of thermodynamics (2pts) 1. Explain the statistical definition of entropy (4pts) 2. Consider a “thermodynamic system” of two dices and let the energy of a certain throw (state of the system) be the sum of the two values of the dices. Calculate the respective entr ...
... 9.1 Entropy in second and third laws of thermodynamics (2pts) 1. Explain the statistical definition of entropy (4pts) 2. Consider a “thermodynamic system” of two dices and let the energy of a certain throw (state of the system) be the sum of the two values of the dices. Calculate the respective entr ...
What is Thermodynamics?
... could be of great value in leading to an understanding of the factors determining the direction of chemical changes. dU = TdS-pdV ...
... could be of great value in leading to an understanding of the factors determining the direction of chemical changes. dU = TdS-pdV ...
Equilibrium Thermodynamics
... - Thermodynamics predicts that the average macroscopic properties of a system in equilibrium are not independent from each other. Therefore, if we measure a subset of these properties, we can calculate the rest of them using thermodynamic relations. - Thermodynamics not only gives the exact descri ...
... - Thermodynamics predicts that the average macroscopic properties of a system in equilibrium are not independent from each other. Therefore, if we measure a subset of these properties, we can calculate the rest of them using thermodynamic relations. - Thermodynamics not only gives the exact descri ...
Biogeochemical cycles and thermodynamics
... Gibbs free energy for a reaction is proportional to the extent of disequilibria. Since G = 0 at equilibrium, G < 0 implies disequilibria. Processes characterized by G > 0 violate the second law of thermodynamics and do not occur spontaneously. Gibbs free energy is expressed in Joules and is a mea ...
... Gibbs free energy for a reaction is proportional to the extent of disequilibria. Since G = 0 at equilibrium, G < 0 implies disequilibria. Processes characterized by G > 0 violate the second law of thermodynamics and do not occur spontaneously. Gibbs free energy is expressed in Joules and is a mea ...
Dr.Eman Zakaria Hegazy Quantum Mechanics and Statistical
... Statistical Thermodynamics Introduction and Definitions Statistical Thermodynamics: is the application of probability theory, which includes mathematical tools for dealing with large populations, to the field of mechanics, which is concerned with the motion of particles when subjected to a force. - ...
... Statistical Thermodynamics Introduction and Definitions Statistical Thermodynamics: is the application of probability theory, which includes mathematical tools for dealing with large populations, to the field of mechanics, which is concerned with the motion of particles when subjected to a force. - ...
Physical Chemistry III
... o To acquire the foundations and terminology which characterize the thermodynamic chemistry of material balances in terms of state functions. o To apply thermodynamic chemistry to the resolution of significant problems such as energy changes in chemical reactions, phase changes, solutions, chemical ...
... o To acquire the foundations and terminology which characterize the thermodynamic chemistry of material balances in terms of state functions. o To apply thermodynamic chemistry to the resolution of significant problems such as energy changes in chemical reactions, phase changes, solutions, chemical ...
PowerPoint Presentation - Chapter 1 Introduction
... called statistical thermodynamics, which can be thought of as a bridge between macroscopic and microscopic properties of systems.[11] Essentially, statistical thermodynamics is an approach to thermodynamics situated upon statistical mechanics, which focuses on the derivation of macroscopic results f ...
... called statistical thermodynamics, which can be thought of as a bridge between macroscopic and microscopic properties of systems.[11] Essentially, statistical thermodynamics is an approach to thermodynamics situated upon statistical mechanics, which focuses on the derivation of macroscopic results f ...
W - Boulder School for Condensed Matter and Materials Physics
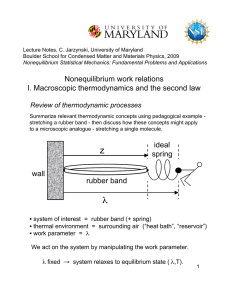
... but keep in mind that the meaning of the quantities involved depends on how we define our system of interest. ...
... but keep in mind that the meaning of the quantities involved depends on how we define our system of interest. ...
The Second Law of Thermodynamics
... 3. The theory emphasizes reversible processes! Yet, real processes are irreversible! ...
... 3. The theory emphasizes reversible processes! Yet, real processes are irreversible! ...
ABCT2772
... values in simple processes b. use the Thermodynamics principles and functions to analysis simple chemical systems and determine the effect of external conditions on their equilibrium positions. c. demonstrate a better understanding on the fundamental principles of reaction rate theories as well as t ...
... values in simple processes b. use the Thermodynamics principles and functions to analysis simple chemical systems and determine the effect of external conditions on their equilibrium positions. c. demonstrate a better understanding on the fundamental principles of reaction rate theories as well as t ...
V α - Springer
... the extensive parameters of any composite system, defined for all equilibrium states and having the following property: The values assumed by the extensive parameters in the absence of an internal constraint are those that maximize the entropy over the manifold of constrained equilibrium states. 3. ...
... the extensive parameters of any composite system, defined for all equilibrium states and having the following property: The values assumed by the extensive parameters in the absence of an internal constraint are those that maximize the entropy over the manifold of constrained equilibrium states. 3. ...
here
... system is unavailable. A mathematically defined thermodynamic function of state, the increase in which gives a measure of the energy of a system which has ceased to be available for work during a certain process: ds = (du + pdv)/T >= dq/T where s is specific entropy; u is specific internal energy; p ...
... system is unavailable. A mathematically defined thermodynamic function of state, the increase in which gives a measure of the energy of a system which has ceased to be available for work during a certain process: ds = (du + pdv)/T >= dq/T where s is specific entropy; u is specific internal energy; p ...
Entropy and the end of it all
... Some physicists believe the second law of thermodynamics implies the world will end with everything being heat, 'the heat death of the universe'. Everybody goes to hell, so to ...
... Some physicists believe the second law of thermodynamics implies the world will end with everything being heat, 'the heat death of the universe'. Everybody goes to hell, so to ...
$doc.title
... In this unit we analyze fundamental concepts of this branch of physics such as thermodynamic systems, state variables and functions, types of thermodynamic processes, etc., and introduce the first and second laws of thermodynamics. ...
... In this unit we analyze fundamental concepts of this branch of physics such as thermodynamic systems, state variables and functions, types of thermodynamic processes, etc., and introduce the first and second laws of thermodynamics. ...