Introduction to Heat Transfer
... derivations. Key terms, such as enthalpy and free energies, as well as experimental variables, such as the coefficient of thermal expansion and compressibility, will be defined. Maxwell relations will then be used to incorporate the experimental variables into expressions for thermodynamic parameter ...
... derivations. Key terms, such as enthalpy and free energies, as well as experimental variables, such as the coefficient of thermal expansion and compressibility, will be defined. Maxwell relations will then be used to incorporate the experimental variables into expressions for thermodynamic parameter ...
15.1,2
... of heat from its surroundings, and 2200 J of work is done by the system on the surroundings. In part b, the system also gains 1500 J of heat, but 2200 J of work is done on the system by the surroundings. In each case, determine the change in the internal energy of the system. ...
... of heat from its surroundings, and 2200 J of work is done by the system on the surroundings. In part b, the system also gains 1500 J of heat, but 2200 J of work is done on the system by the surroundings. In each case, determine the change in the internal energy of the system. ...
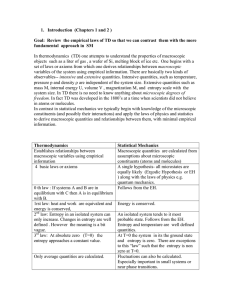
1. Introduction (Chapters 1 and 2 ) Goal: Review the empirical laws
... set of laws or axioms from which one derives relationships between macroscopic variables of the system using empirical information. There are basically two kinds of observables-- intensive and extensive quantities. Intensive quantities, such as temperature, pressure p and density are independent o ...
... set of laws or axioms from which one derives relationships between macroscopic variables of the system using empirical information. There are basically two kinds of observables-- intensive and extensive quantities. Intensive quantities, such as temperature, pressure p and density are independent o ...
Lecture 1 1 Overview
... We will use V for extensive, Ṽ for molar, V̂ for specific, and V i for partial molar. 3. Gibbs Phase Rule. We observe that there are many variables for a given system, e.g., pressure, density, internal energy, viscosity, thermal conductivity, composition, volume, etc. How many of these properties d ...
... We will use V for extensive, Ṽ for molar, V̂ for specific, and V i for partial molar. 3. Gibbs Phase Rule. We observe that there are many variables for a given system, e.g., pressure, density, internal energy, viscosity, thermal conductivity, composition, volume, etc. How many of these properties d ...
any physical system, whether or not it can exchange energy and
... Open system A system, over the border of which both energy and mass can be transmitted. ...
... Open system A system, over the border of which both energy and mass can be transmitted. ...
10CH301 - Karunya University
... Unit III Phase rule: Gibbs phase rule and phase equilibria – Degree of freedom – One component system – Water system – Sulphur system – Carbon-di-oxide system – Carbon system – Helium System – Two components system – Reduced phase rule – Lead – silver system – Pattinson’s process – Ferric chloride – ...
... Unit III Phase rule: Gibbs phase rule and phase equilibria – Degree of freedom – One component system – Water system – Sulphur system – Carbon-di-oxide system – Carbon system – Helium System – Two components system – Reduced phase rule – Lead – silver system – Pattinson’s process – Ferric chloride – ...
název projektu
... 4 laws of thermodynamics summarize the most important facts of thermodynamics. They: •define fundamental physical quantities, such as temperature, energy, and entropy •describe thermodynamic systems by virtue of these quantities •describe the transfer of energy as heat and work in thermodynamic proc ...
... 4 laws of thermodynamics summarize the most important facts of thermodynamics. They: •define fundamental physical quantities, such as temperature, energy, and entropy •describe thermodynamic systems by virtue of these quantities •describe the transfer of energy as heat and work in thermodynamic proc ...
Physical chemistry 1
... 4. Describe simple electrochemical and thermodynamic measurements; 5. Experimentally determine certain physical variables; 6. Apply calculation in solving physical and chemical problems. ...
... 4. Describe simple electrochemical and thermodynamic measurements; 5. Experimentally determine certain physical variables; 6. Apply calculation in solving physical and chemical problems. ...
Chapter 1 Introduction and Definition of Terms
... independent of the path of the change. e.g. T, V, P, U, H, S, G. 7. Equation of State A mathematical relationship between the state functions. Example: Consider the volume V of a fixed quantity of a pure gas as a property, the value of which is dependent on the values of P and T. The relationship be ...
... independent of the path of the change. e.g. T, V, P, U, H, S, G. 7. Equation of State A mathematical relationship between the state functions. Example: Consider the volume V of a fixed quantity of a pure gas as a property, the value of which is dependent on the values of P and T. The relationship be ...
Word - The University of British Columbia
... processes. At the end of the semester, students are expected to: ...
... processes. At the end of the semester, students are expected to: ...
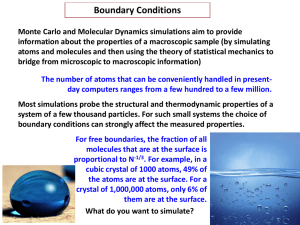
Frenkel and Smit / Chandler
... properties (the Boltzmann factor was derived assuming equilibrium). What about non-equilibrium properties? Example: relaxation rate by which a system reaches equilibrium from a prepared non-equilibrium state (e.g. begin with all reactions, no products, and allow a chemical reaction to proceed) Our d ...
... properties (the Boltzmann factor was derived assuming equilibrium). What about non-equilibrium properties? Example: relaxation rate by which a system reaches equilibrium from a prepared non-equilibrium state (e.g. begin with all reactions, no products, and allow a chemical reaction to proceed) Our d ...
эритмалар. эритмалар назарияси. эритмаларнинг хоссалари
... In such conditions, according to the first law of thermodynamics, the total amount of heat supplied to the system spent to increase the internal energy: ...
... In such conditions, according to the first law of thermodynamics, the total amount of heat supplied to the system spent to increase the internal energy: ...
ACS_Thermodynamics_Exam_1981
... a. B and C are close to what we want but are written with the wrong order. Since we just stated that state functions have exact differentials we should look something that signifies this. Answer A is saying that a closed path (the loop in the integral symbol) is zero. In other words the function is ...
... a. B and C are close to what we want but are written with the wrong order. Since we just stated that state functions have exact differentials we should look something that signifies this. Answer A is saying that a closed path (the loop in the integral symbol) is zero. In other words the function is ...
CHEM 211: Physical Chemistry
... Objectives: After taking this course students are expected to understand - how energy is exchanged between the system and surroundings under different conditions. - how entropy and Gibbs free energy can be used to predict the direction of the spontaneous change and estimate the position of equilibri ...
... Objectives: After taking this course students are expected to understand - how energy is exchanged between the system and surroundings under different conditions. - how entropy and Gibbs free energy can be used to predict the direction of the spontaneous change and estimate the position of equilibri ...
Thermodynamics
... under the pressure-volume curve which represents the process taking place. The more general expression for work done is: ...
... under the pressure-volume curve which represents the process taking place. The more general expression for work done is: ...
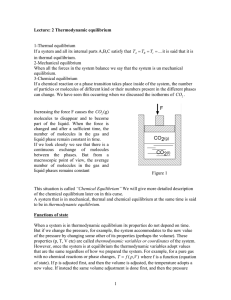
1 Lecture: 2 Thermodynamic equilibrium 1
... constant for a conservative system. If we include all the variables that describe the processes, all systems are conservative. It follows that the energy is always conserved. We consider a system “A” surrounded by the rest of the universe, and we say that the system has a certain amount of energy U ...
... constant for a conservative system. If we include all the variables that describe the processes, all systems are conservative. It follows that the energy is always conserved. We consider a system “A” surrounded by the rest of the universe, and we say that the system has a certain amount of energy U ...