Thermodynamic Concep..
... solvent. Thus, in essentially all biochemical reactions, water is always at its standard state (which will mean we can ignore it in our thermodynamic calculations for the most part). You should also note that in biochemistry, unlike chemistry, the standard state of H+ is 10-7 M (pH 7.0) rather than ...
... solvent. Thus, in essentially all biochemical reactions, water is always at its standard state (which will mean we can ignore it in our thermodynamic calculations for the most part). You should also note that in biochemistry, unlike chemistry, the standard state of H+ is 10-7 M (pH 7.0) rather than ...
Internal Energy Work Heat
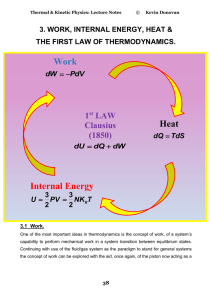
... In the equation, đW = -PdV , where an infinitesimal amount of work, đW has been performed (in this case on the system) the bar through the đ indicates the fact that the amount of work for a given volume change depends on the path chosen ie how the change dV is made. The change from an initial equili ...
... In the equation, đW = -PdV , where an infinitesimal amount of work, đW has been performed (in this case on the system) the bar through the đ indicates the fact that the amount of work for a given volume change depends on the path chosen ie how the change dV is made. The change from an initial equili ...
THERMODYNAMICS LECTURE NOTES
... Everything external to the control volume is the surroundings with the separation given by the control surface. The surface may be open or closed to mass flows and it may have flows from energy in terms of heat transfer and work across it. The boundaries may be moveable or stationary. In the case of ...
... Everything external to the control volume is the surroundings with the separation given by the control surface. The surface may be open or closed to mass flows and it may have flows from energy in terms of heat transfer and work across it. The boundaries may be moveable or stationary. In the case of ...
Statistical Physics Problem Sets 3–4: Kinetic Theory xford hysics
... 3.10 A gas is a mixture of H2 and HD in the proportion 7000:1. As the gas effuses through a small hole from a vessel at constant temperature into a vacuum, the composition of the remaining mixture changes. By what factor will the pressure in the vessel have fallen when the remaining mixture consists ...
... 3.10 A gas is a mixture of H2 and HD in the proportion 7000:1. As the gas effuses through a small hole from a vessel at constant temperature into a vacuum, the composition of the remaining mixture changes. By what factor will the pressure in the vessel have fallen when the remaining mixture consists ...
Lesson 5 Energy Transfer Energy Transfer Notes The movement of
... down and the cold water particle sped up. This transfer of kinetic energy continued until all of the water particles throughout the volume of water were moving at about the same speed. The final average speed of the particles was faster than the particles in the original cold water and slower than t ...
... down and the cold water particle sped up. This transfer of kinetic energy continued until all of the water particles throughout the volume of water were moving at about the same speed. The final average speed of the particles was faster than the particles in the original cold water and slower than t ...
Thermodynamics
... cycle occurs when a system is taken through a series of different states, and finally returned to its initial state. In the process of going through this cycle, the system may perform work on its surroundings, thereby acting as a heat engine. A heat engine acts by transferring energy from a warm reg ...
... cycle occurs when a system is taken through a series of different states, and finally returned to its initial state. In the process of going through this cycle, the system may perform work on its surroundings, thereby acting as a heat engine. A heat engine acts by transferring energy from a warm reg ...
thermodynamics type 1
... of quantities of heat & work. It may be defined as the branch of science which deals with energy changes associated with various physical & chemical processes. The entire formulation of thermodynamics is based on a few (Three) fundamental laws which have been established on the basis of human experi ...
... of quantities of heat & work. It may be defined as the branch of science which deals with energy changes associated with various physical & chemical processes. The entire formulation of thermodynamics is based on a few (Three) fundamental laws which have been established on the basis of human experi ...
EQATION OF STATE IN FORM WHICH RELATES MOL FRACTION
... Most people including specialists believe that a model was made that absolutely describes the thermodynamic system consisted of an ideal gas. Indeed the ideal gas state equation connects well all the parameters in an ideal gas system. But if we try to solve the following problem: A thermodynamic sys ...
... Most people including specialists believe that a model was made that absolutely describes the thermodynamic system consisted of an ideal gas. Indeed the ideal gas state equation connects well all the parameters in an ideal gas system. But if we try to solve the following problem: A thermodynamic sys ...
Thermal Energy and Heat + Conservation of Energy
... movement of particles from one location to another. Thermal energy transfer by convection usually occurs in gases and liquids. During convection, the movement of the particles forms a current, which is a flow, from one place to another in one direction. Liquid water has a high heat capacity which ...
... movement of particles from one location to another. Thermal energy transfer by convection usually occurs in gases and liquids. During convection, the movement of the particles forms a current, which is a flow, from one place to another in one direction. Liquid water has a high heat capacity which ...
Second Law of Thermodynamics
... contained n moles of an ideal gas, showing first that it was reversible, and most importantly that—regardless of the specific heat of the gas—it had limited efficiency, defined as e = W /Qh , where W is the net work done by the engine and Qh is the quantity of heat put into the engine at a (high) te ...
... contained n moles of an ideal gas, showing first that it was reversible, and most importantly that—regardless of the specific heat of the gas—it had limited efficiency, defined as e = W /Qh , where W is the net work done by the engine and Qh is the quantity of heat put into the engine at a (high) te ...
Document
... to rise (line DA) until the temperature reaches its original value (TA = TB). Calculate (a) the total work done by the gas in the process BDA, and (b) the total heat flow into the gas. ...
... to rise (line DA) until the temperature reaches its original value (TA = TB). Calculate (a) the total work done by the gas in the process BDA, and (b) the total heat flow into the gas. ...
S8P2 Students will be familiar with the forms and transformations of
... • Energy cannot be created or destroyed, but only changed from one form into another. • Transformations of energy usually release some energy typically in the form of heat. • Temperature changes as heat is transferred from a hotter object to a colder one. • Heat transfer occurs by conduction, convec ...
... • Energy cannot be created or destroyed, but only changed from one form into another. • Transformations of energy usually release some energy typically in the form of heat. • Temperature changes as heat is transferred from a hotter object to a colder one. • Heat transfer occurs by conduction, convec ...
The Formation and Structure of Stars Chapter 10
... • The first two laws of stellar structure have some thing in common—they are both what astronomers and physicists call ...
... • The first two laws of stellar structure have some thing in common—they are both what astronomers and physicists call ...