Heat and Properties of Matter
... Figure 1.2: Explanation of Bragg’s law. Two incident X-rays are reflected by the planes. The triangles denote the necessary conditions for constructive interference. Lets consider the arrangement on fig. 1.2.1. An X-ray which reflects from the surface of a substance has travelled less distance than ...
... Figure 1.2: Explanation of Bragg’s law. Two incident X-rays are reflected by the planes. The triangles denote the necessary conditions for constructive interference. Lets consider the arrangement on fig. 1.2.1. An X-ray which reflects from the surface of a substance has travelled less distance than ...
PowerPoint Lecture
... • If you want to heat the 3 m cubic room by 10ºC with a 1000 W space heater, how long will it take? We know from before that the room needs to have 360,000 J added to it, so at 1000 W = 1000 J/s this will take 360 seconds, or six minutes. But: the walls need to be warmed up too, so it will actually ...
... • If you want to heat the 3 m cubic room by 10ºC with a 1000 W space heater, how long will it take? We know from before that the room needs to have 360,000 J added to it, so at 1000 W = 1000 J/s this will take 360 seconds, or six minutes. But: the walls need to be warmed up too, so it will actually ...
Using a Graduated Cylinder
... 1. If each graduated cylinder pictured were filled to the top graduation, what would the volume be? Write the answer next to 250mL each letter. 2. When most liquids are placed in tall, narrow, glass containers, they creep up the walls of the container. This results in the surface of the liquid appea ...
... 1. If each graduated cylinder pictured were filled to the top graduation, what would the volume be? Write the answer next to 250mL each letter. 2. When most liquids are placed in tall, narrow, glass containers, they creep up the walls of the container. This results in the surface of the liquid appea ...
Action-at-a-Distance Forces Contact Forces
... Energy may be stored in a number of different ways. In gravitational potential energy, energy is stored in the object’s height. Chemical potential energy occurs when energy is stored in the object’s molecules. Energy may be stored in a stressed object. This is mechanical potential energy. Finally, ...
... Energy may be stored in a number of different ways. In gravitational potential energy, energy is stored in the object’s height. Chemical potential energy occurs when energy is stored in the object’s molecules. Energy may be stored in a stressed object. This is mechanical potential energy. Finally, ...
Thermodynamic Laws, Entropy and CPH Theory
... Also, a system (of atoms or molecules) is at basic level energy, if it loses all its energy and its elements keep their properties. When a system is at basic level energy, its charge particles are not able work on each other, so system does not emit heat energy. When a system is at basic level energ ...
... Also, a system (of atoms or molecules) is at basic level energy, if it loses all its energy and its elements keep their properties. When a system is at basic level energy, its charge particles are not able work on each other, so system does not emit heat energy. When a system is at basic level energ ...
b - UCSC Physics
... A car slows down as a result of air resistance. Which is true? • A. The car’s kinetic energy decreases. • B. Heat is generated. • C. The energy of the car/road/air system is constant. • D. all of the above • E. none of the above ...
... A car slows down as a result of air resistance. Which is true? • A. The car’s kinetic energy decreases. • B. Heat is generated. • C. The energy of the car/road/air system is constant. • D. all of the above • E. none of the above ...
Lecture VIII_IX
... temperature and expansion L is given by f(T,L) = aT(L-L0) where a and L0 are constants. • (a)Use Maxwell relations to determine the entropy and enthalpy at constant T and p. • (b) If you adiabatically stretch a rubber band by small amount, its temperature increases but volume does not change. Derive ...
... temperature and expansion L is given by f(T,L) = aT(L-L0) where a and L0 are constants. • (a)Use Maxwell relations to determine the entropy and enthalpy at constant T and p. • (b) If you adiabatically stretch a rubber band by small amount, its temperature increases but volume does not change. Derive ...
Heat Capacity - Uplift North Hills Prep
... • The interior of roasted meat can never reach temperatures higher than the boiling point of water until all the water is cooked out of it, at which point it would resemble shoe leather. The outside is quickly dried out, however, and can reach the temperature of the surrounding cooking medium. • Coc ...
... • The interior of roasted meat can never reach temperatures higher than the boiling point of water until all the water is cooked out of it, at which point it would resemble shoe leather. The outside is quickly dried out, however, and can reach the temperature of the surrounding cooking medium. • Coc ...
A thermodynamic system is one that interacts and exchanges
... move from cooler to warmer areas, it is going against what is “natural”, so the system must put in some work for it to happen. ...
... move from cooler to warmer areas, it is going against what is “natural”, so the system must put in some work for it to happen. ...
Physical Chemistry
... If a system changes from a to b, then: -ve G means that the system or process can ...
... If a system changes from a to b, then: -ve G means that the system or process can ...
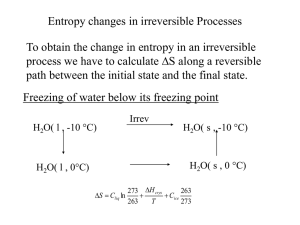
Part III: Second Law of Thermodynamics
... law of thermodynamics. We show later in Part III that the reverse processes discussed above violate the second law of thermodynamics. This violation is easily detected with the help of a property, called entropy, defined in the next part. A process will not occur unless it satisfies both the first a ...
... law of thermodynamics. We show later in Part III that the reverse processes discussed above violate the second law of thermodynamics. This violation is easily detected with the help of a property, called entropy, defined in the next part. A process will not occur unless it satisfies both the first a ...
Тепломассообмен
... 1. To understand matter it is necessary to consider its molecules, which are in constant motion, colliding and rebounding not unlike billiard balls. To describe matter the history of each molecule must be known. This requires knowing each molecule’s velocity and acceleration which is quite impossibl ...
... 1. To understand matter it is necessary to consider its molecules, which are in constant motion, colliding and rebounding not unlike billiard balls. To describe matter the history of each molecule must be known. This requires knowing each molecule’s velocity and acceleration which is quite impossibl ...
Topic 6 – Energy and the Future
... THE EARTH’S TEMPERATURE For a system to stay at a constant temperature it must absorb the same amount of power as it radiates (i.e it must take in the same amount of energy as it gives out) E.g if a pool at 27°C radiates 1200W, the heating system must transfer 1200W to the pool for its temperature t ...
... THE EARTH’S TEMPERATURE For a system to stay at a constant temperature it must absorb the same amount of power as it radiates (i.e it must take in the same amount of energy as it gives out) E.g if a pool at 27°C radiates 1200W, the heating system must transfer 1200W to the pool for its temperature t ...
Efficiency
... Kinetic energy and work of a moving object • Equal to the work required to bring it from rest to that speed, or the work the object can do while being brought to rest • In equation form: net force distance kinetic energy, or Fd 1/2 mv2 ...
... Kinetic energy and work of a moving object • Equal to the work required to bring it from rest to that speed, or the work the object can do while being brought to rest • In equation form: net force distance kinetic energy, or Fd 1/2 mv2 ...