Unit 4 Study guide
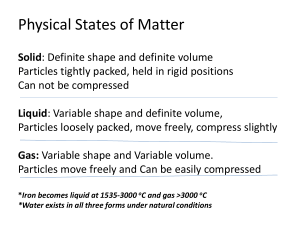
... 52. How many kilojoules of heat must be transferred to a 670-g aluminum pan to raise its temperature from 32◦C to 250◦C? The specific heat of aluminum is 0.96 J/g·◦C. 53. ________ is a measure of the average kinetic energy of all the particles within an object. 54. The energy transferred between the ...
... 52. How many kilojoules of heat must be transferred to a 670-g aluminum pan to raise its temperature from 32◦C to 250◦C? The specific heat of aluminum is 0.96 J/g·◦C. 53. ________ is a measure of the average kinetic energy of all the particles within an object. 54. The energy transferred between the ...
Unit 4: Energy and Heat Study Guide
... 52. How many kilojoules of heat must be transferred to a 670-g aluminum pan to raise its temperature from 32◦C to 250◦C? The specific heat of aluminum is 0.96 J/g·◦C. 53. ________ is a measure of the average kinetic energy of all the particles within an object. 54. The energy transferred between the ...
... 52. How many kilojoules of heat must be transferred to a 670-g aluminum pan to raise its temperature from 32◦C to 250◦C? The specific heat of aluminum is 0.96 J/g·◦C. 53. ________ is a measure of the average kinetic energy of all the particles within an object. 54. The energy transferred between the ...
Document
... 52. How many kilojoules of heat must be transferred to a 670-g aluminum pan to raise its temperature from 32◦C to 250◦C? The specific heat of aluminum is 0.96 J/g·◦C. 53. ________ is a measure of the average kinetic energy of all the particles within an object. 54. The energy transferred between the ...
... 52. How many kilojoules of heat must be transferred to a 670-g aluminum pan to raise its temperature from 32◦C to 250◦C? The specific heat of aluminum is 0.96 J/g·◦C. 53. ________ is a measure of the average kinetic energy of all the particles within an object. 54. The energy transferred between the ...
Final Exam Review Sheet (Physics Semester)
... How do you calculate gravitational potential energy? What is kinetic energy? How do you calculate kinetic energy? What is mechanical energy and how does it relate to PE and KE? Can you explain how potential energy and kinetic energy are related for different examples, such as, a roller coaster, pend ...
... How do you calculate gravitational potential energy? What is kinetic energy? How do you calculate kinetic energy? What is mechanical energy and how does it relate to PE and KE? Can you explain how potential energy and kinetic energy are related for different examples, such as, a roller coaster, pend ...
On the Foundations of Classical Thermodynamics, and the Tolman
... into the colder surroundings until the body has cooled down and the heat gradient has vanished. After uniformity of hotness has been established, no more changes will occur, and at this point the body is said to have reached thermal equilibrium with its surroundings. The meaning of the term ”hotness ...
... into the colder surroundings until the body has cooled down and the heat gradient has vanished. After uniformity of hotness has been established, no more changes will occur, and at this point the body is said to have reached thermal equilibrium with its surroundings. The meaning of the term ”hotness ...
The EoS, together with the thermodynamic equation, allows to
... including the ionization energy. P and E clearly depend only on the temperature and density. For real calculation, of course, all species, energy levels, and reactions must be considered. For example, one also has the ionization-recombination for Helium at high temperatures. The presence of such zon ...
... including the ionization energy. P and E clearly depend only on the temperature and density. For real calculation, of course, all species, energy levels, and reactions must be considered. For example, one also has the ionization-recombination for Helium at high temperatures. The presence of such zon ...
File
... = 1.5) is taken through an adiabatic process in which the volume is compressed from 1600cc to 400cc. If the initial pressure is 150kpa, what is the final pressure and how much work is done on the gas in the process? ...
... = 1.5) is taken through an adiabatic process in which the volume is compressed from 1600cc to 400cc. If the initial pressure is 150kpa, what is the final pressure and how much work is done on the gas in the process? ...
P K P K K K P P
... 14. Chemical Energy: energy stored in the bonds of atoms and molecules Example: biomass, petroleum (gasoline), natural gas 15. Nuclear Energy: energy stored in the nucleus of an atom Example: the sun (fusion), nuclear (fission) in power plants List two ways nuclear energy is produced: fusion and fis ...
... 14. Chemical Energy: energy stored in the bonds of atoms and molecules Example: biomass, petroleum (gasoline), natural gas 15. Nuclear Energy: energy stored in the nucleus of an atom Example: the sun (fusion), nuclear (fission) in power plants List two ways nuclear energy is produced: fusion and fis ...
Energy Target Review - Scott County Schools
... transferred. The total amount of energy in a system remains constant. For example, a bouncing basketball. When off the ground, the ball has gravitational potential energy. When dropped, the gravitational energy gets less and less as it gets closer to the ground, and that is converted into mechanical ...
... transferred. The total amount of energy in a system remains constant. For example, a bouncing basketball. When off the ground, the ball has gravitational potential energy. When dropped, the gravitational energy gets less and less as it gets closer to the ground, and that is converted into mechanical ...
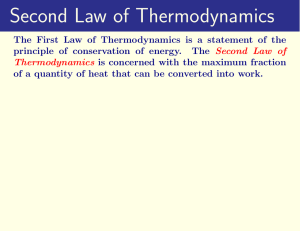
Chapter 6: Entropy and the Laws of Thermodynamics
... situation to a nonequilibrium one, and energy must be transferred to the system. A compressed air tank with its vale open is approaching equilibrium. Equilibrium occurs when the pressure inside the tank is the same as the pressure outside. To recompress the air in the tank, work is required. Work an ...
... situation to a nonequilibrium one, and energy must be transferred to the system. A compressed air tank with its vale open is approaching equilibrium. Equilibrium occurs when the pressure inside the tank is the same as the pressure outside. To recompress the air in the tank, work is required. Work an ...