Lessons 9
... Thermochemistry is the study of energy changes involved in chemical reactions. These changes can be physical (i.e. melting ice,) chemical (rusting iron) or nuclear (nuclear fusion reactions in the Sun). All energy transformations are governed by the 1st Law of Thermodynamics, which states that the t ...
... Thermochemistry is the study of energy changes involved in chemical reactions. These changes can be physical (i.e. melting ice,) chemical (rusting iron) or nuclear (nuclear fusion reactions in the Sun). All energy transformations are governed by the 1st Law of Thermodynamics, which states that the t ...
photosynthesis: the green miracle
... intelligence and artistry. When a leaf is viewed from the outside, its shape and structures display a design aimed at a particular purpose. For example, the leaf needs to remain flat in order to receive the maximum amount of solar rays but for that to be possible, the leaf must have a special design ...
... intelligence and artistry. When a leaf is viewed from the outside, its shape and structures display a design aimed at a particular purpose. For example, the leaf needs to remain flat in order to receive the maximum amount of solar rays but for that to be possible, the leaf must have a special design ...
kcse chemistry questions
... Give a reason why calcium hydroxide solution is used to detect the presence of carbon dioxide gas while sodium hydroxide in NOT? a compound C4H10O_ is oxidized by excess acidified potassium permanganate to form another compound C4H8O2. The same compound C4H10O reacts with potassium to produce hydrog ...
... Give a reason why calcium hydroxide solution is used to detect the presence of carbon dioxide gas while sodium hydroxide in NOT? a compound C4H10O_ is oxidized by excess acidified potassium permanganate to form another compound C4H8O2. The same compound C4H10O reacts with potassium to produce hydrog ...
Higher Chemistry Resources Guide - Glow Blogs
... 3. a visually attractive and colourful reaction between sodium thiosulfate and hydrogen peroxide in the presence of universal indicator 4. the attention-grabbing classic cannon fire experiment ...
... 3. a visually attractive and colourful reaction between sodium thiosulfate and hydrogen peroxide in the presence of universal indicator 4. the attention-grabbing classic cannon fire experiment ...
PDF - mockies – Mockiesgateacademy
... aims of alchemy that emerged with time were the quest for the elixir of life (the drinking of which would endue the alchemist with immortality), and the search for the philosopher’s stone, which would turn base metals into gold. Improbable as these ideas might seem today, the alchemists continued th ...
... aims of alchemy that emerged with time were the quest for the elixir of life (the drinking of which would endue the alchemist with immortality), and the search for the philosopher’s stone, which would turn base metals into gold. Improbable as these ideas might seem today, the alchemists continued th ...
Higher Chemistry Resources Guide - Glow Blogs
... 3. a visually attractive and colourful reaction between sodium thiosulfate and hydrogen peroxide in the presence of universal indicator 4. the attention-grabbing classic cannon fire experiment ...
... 3. a visually attractive and colourful reaction between sodium thiosulfate and hydrogen peroxide in the presence of universal indicator 4. the attention-grabbing classic cannon fire experiment ...
CHE 1031 Lab Manual
... Knowledge in chemistry, as in all the physical sciences, is obtained initially from performing experiments in a laboratory. It is in the laboratory that facts are discovered and concepts, ideas and theorie ...
... Knowledge in chemistry, as in all the physical sciences, is obtained initially from performing experiments in a laboratory. It is in the laboratory that facts are discovered and concepts, ideas and theorie ...
Mineralization of Drugs in Aqueous Medium by Advanced Oxidation
... as emerging pollutants. This pollution can be due to different sources involving emission from production sites, direct disposal of overplus drugs in households, excretion after drug administration to humans and animals, treatments throughout the water in fish and other animal farms and inadequate t ...
... as emerging pollutants. This pollution can be due to different sources involving emission from production sites, direct disposal of overplus drugs in households, excretion after drug administration to humans and animals, treatments throughout the water in fish and other animal farms and inadequate t ...
Avogadro`s Number, Moles and Molar Mass
... "measure"). Stoichiometry calculations are based on the fact that atoms are conserved. They cannot be destroyed or created. Numbers and kinds of atoms before and after the reactions are always the same. This is the Law of Conservation of mass and is why chemical reactions must be balanced. The molar ...
... "measure"). Stoichiometry calculations are based on the fact that atoms are conserved. They cannot be destroyed or created. Numbers and kinds of atoms before and after the reactions are always the same. This is the Law of Conservation of mass and is why chemical reactions must be balanced. The molar ...
Lecture 25 Notes
... The hydronium ion A hydrogen ion (H+) does not exist by itself in an aqueous solution • In water, H+ combines with a polar H2O molecule to form a hydrated hydrogen ion (H3O+) called a hydronium ion ...
... The hydronium ion A hydrogen ion (H+) does not exist by itself in an aqueous solution • In water, H+ combines with a polar H2O molecule to form a hydrated hydrogen ion (H3O+) called a hydronium ion ...
Mole Concept
... of B to give c moles of C and d moles of D. This means, for instance, that Y moles of A will require Y mol A x (b mol B / a mol A) = Yb/a mol B, since the stoichiometric coefficients are conversion factors between different species. We define 1 mol of this reaction as the process of mixing a mol of ...
... of B to give c moles of C and d moles of D. This means, for instance, that Y moles of A will require Y mol A x (b mol B / a mol A) = Yb/a mol B, since the stoichiometric coefficients are conversion factors between different species. We define 1 mol of this reaction as the process of mixing a mol of ...
Final Review 3-8 Answers_2
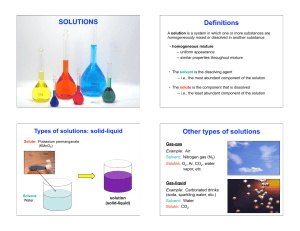
... 12. A substance was dissolved in water to form a conducting solution that does not affect litmus. This evidence indicates a/an a) acid substance that has ionized in solution b) neutral ionic substance that has dissociated in solution c) base substance that has dissociated in solution d) base substan ...
... 12. A substance was dissolved in water to form a conducting solution that does not affect litmus. This evidence indicates a/an a) acid substance that has ionized in solution b) neutral ionic substance that has dissociated in solution c) base substance that has dissociated in solution d) base substan ...
Appendix
... Sodium Vapor Lighting The flame test for sodium shows two bright lines at 589.0 and 589.6 nm, which is the yellow range of the emission spectrum. Sodium can be vaporized at high temperatures in a sealed tube and made to give off light using two electrodes connected to a power source. Sodium vapor l ...
... Sodium Vapor Lighting The flame test for sodium shows two bright lines at 589.0 and 589.6 nm, which is the yellow range of the emission spectrum. Sodium can be vaporized at high temperatures in a sealed tube and made to give off light using two electrodes connected to a power source. Sodium vapor l ...
Chapter 3 Mass Relationships in Chemical Reactions 1
... Chapter 3 Mass Relationships in Chemical Reactions ...
... Chapter 3 Mass Relationships in Chemical Reactions ...
kinetic characterisation of catalysts for methanol synthesis
... manufacture TMC-3/1 a catalyst with the composition of Cu (wt%) -50, ZnO (wt%)-25, Al2O3 (wt%)10%. It has the form of tablets with the dimensions of 3.5-4.5 mm. AlVIGO produces Cu/ZnO/Al2O3 called CHM-Y. The catalyst contains Cu (wt%) – 53; ZnO (wt%)-26, Al2O3 (wt%)-5.5%. The density of the catalyst ...
... manufacture TMC-3/1 a catalyst with the composition of Cu (wt%) -50, ZnO (wt%)-25, Al2O3 (wt%)10%. It has the form of tablets with the dimensions of 3.5-4.5 mm. AlVIGO produces Cu/ZnO/Al2O3 called CHM-Y. The catalyst contains Cu (wt%) – 53; ZnO (wt%)-26, Al2O3 (wt%)-5.5%. The density of the catalyst ...
Theoretical Study of Gas-Phase Reactions of Fe(CO)5 with OH
... different basis sets (II and II++) have been used in this study. Basis set II uses a small-core effective core potential (ECP) with a (441/2111/41) split-valence basis set for Fe, which is derived from the (55/5/5) minimal basis set optimized by Hay and Wadt,22 and 6-31G(d,p) all-electron basis sets ...
... different basis sets (II and II++) have been used in this study. Basis set II uses a small-core effective core potential (ECP) with a (441/2111/41) split-valence basis set for Fe, which is derived from the (55/5/5) minimal basis set optimized by Hay and Wadt,22 and 6-31G(d,p) all-electron basis sets ...
Chemical Dynamics at Surfaces
... Irving Langmuir was born in Brooklyn, New York, on January 31, 1881, as the third of four sons of Charles Langmuir and Sadie, neé Comings. His early education was obtained in various schools and institutes in the USA, and in Paris (1892-1895). He graduated as a metallurgical engineer from the School ...
... Irving Langmuir was born in Brooklyn, New York, on January 31, 1881, as the third of four sons of Charles Langmuir and Sadie, neé Comings. His early education was obtained in various schools and institutes in the USA, and in Paris (1892-1895). He graduated as a metallurgical engineer from the School ...
ExamView - 1984 AP Chemistry Exam.tst
... (1) Test Questions are Copyright © 1984-2002 by College Entrance Examination Board, Princeton, NJ. All rights reserved. For face-to-face teaching purposes, classroom teachers are permitted to reproduce the questions. Web or Mass distribution prohibited. (2) AP® is a registered trademark of the Colle ...
... (1) Test Questions are Copyright © 1984-2002 by College Entrance Examination Board, Princeton, NJ. All rights reserved. For face-to-face teaching purposes, classroom teachers are permitted to reproduce the questions. Web or Mass distribution prohibited. (2) AP® is a registered trademark of the Colle ...
Document
... This publication is copyright Learning Materials Production, Open Training and Education Network – Distance Education, NSW Department of Education and Training, however it may contain material from other sources which is not owned by Learning Materials Production. Learning Materials Production would ...
... This publication is copyright Learning Materials Production, Open Training and Education Network – Distance Education, NSW Department of Education and Training, however it may contain material from other sources which is not owned by Learning Materials Production. Learning Materials Production would ...
Water splitting

Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in the United States. In photosynthesis, water splitting donates electrons to power the electron transport chain in photosystem II.