Solutions
... Supercritical fluids (carbon dioxide, ethane, trifluoromethane (fluoroform), sulfur hexafluoride, dinitrogen oxide (nitrous oxide), carbon disulphide). Supercritical fluids show a dissolving power quickly increasing with pressure (up to a limit). Pure supercritical carbon dioxide is a relatively non ...
... Supercritical fluids (carbon dioxide, ethane, trifluoromethane (fluoroform), sulfur hexafluoride, dinitrogen oxide (nitrous oxide), carbon disulphide). Supercritical fluids show a dissolving power quickly increasing with pressure (up to a limit). Pure supercritical carbon dioxide is a relatively non ...
New Visible-Light Active Semiconductors
... whole solar energy spectrum that radiates the earth, UV irradiation only represents 4 %. In the same way, other semiconductors such as ZnO, Fe2O3, CdS, ZnS, Nb2O5, Ta2O5, and BiTaO4 have been reported with excellent performance as photocatalysts, among others. In particular, oxides with perovskite s ...
... whole solar energy spectrum that radiates the earth, UV irradiation only represents 4 %. In the same way, other semiconductors such as ZnO, Fe2O3, CdS, ZnS, Nb2O5, Ta2O5, and BiTaO4 have been reported with excellent performance as photocatalysts, among others. In particular, oxides with perovskite s ...
Recycling and Chemical Mathematics
... The Earth is the largest materially closed system that humans occupy. The second largest is a 3.15-acre greenhouse in Oracle, Arizona. It has been called Biosphere 2, the “2” being in recognition of the fact that the Earth is Biosphere 1. The original goal of the project was to determine whether a l ...
... The Earth is the largest materially closed system that humans occupy. The second largest is a 3.15-acre greenhouse in Oracle, Arizona. It has been called Biosphere 2, the “2” being in recognition of the fact that the Earth is Biosphere 1. The original goal of the project was to determine whether a l ...
2008 FALL Semester Midterm Examination For
... zwitterions, rather than the amine group, which is already protonated. ...
... zwitterions, rather than the amine group, which is already protonated. ...
AP Chemistry Lab Manual
... someone who is unfamiliar with your work may be using this notebook to evaluate your lab experience in chemistry. When you explain your work, list your data, calculate values and answer questions, be sure that the meaning will be obvious to anyone who reads your notebook. Guidelines for the notebook ...
... someone who is unfamiliar with your work may be using this notebook to evaluate your lab experience in chemistry. When you explain your work, list your data, calculate values and answer questions, be sure that the meaning will be obvious to anyone who reads your notebook. Guidelines for the notebook ...
HSC Chemistry Syllabus Notes 2007
... memorise the content. Secondly, you need to understand the concepts. Questions do not always ask you to recall a memorised slab of information, they sometimes require you to think critically and solve problems. To be able to do this you need to have a clear and deep understanding of the chemistry. T ...
... memorise the content. Secondly, you need to understand the concepts. Questions do not always ask you to recall a memorised slab of information, they sometimes require you to think critically and solve problems. To be able to do this you need to have a clear and deep understanding of the chemistry. T ...
1.8 M - Thierry Karsenti
... Most chemical reactions and virtually all biological processes take place not between pure solids, liquids or gases, but rather among ions and molecules dissolved in water or other solvents (i.e. in solution). In this module we will therefore examine the various types of solutions and their properti ...
... Most chemical reactions and virtually all biological processes take place not between pure solids, liquids or gases, but rather among ions and molecules dissolved in water or other solvents (i.e. in solution). In this module we will therefore examine the various types of solutions and their properti ...
Quarter 1
... It means that ½ of the atoms are isotopes It is the time it takes for ½ of the isotope atoms to decay into another material It is ½ of the age of the isotope It is ½ of the atomic mass of the atom ...
... It means that ½ of the atoms are isotopes It is the time it takes for ½ of the isotope atoms to decay into another material It is ½ of the age of the isotope It is ½ of the atomic mass of the atom ...
Fluorinated Butatrienes - diss.fu-berlin.de
... Es war nicht möglich, neue Tetrafluorbutatrien-Metallkomplexe zu synthetisieren. Dies ist ...
... Es war nicht möglich, neue Tetrafluorbutatrien-Metallkomplexe zu synthetisieren. Dies ist ...
Part A Completion
... ________ 8. The half-cell that has a greater tendency to acquire electrons will be the one in which oxidation occurs. ________ 9. In an electrochemical cell, the hydrogen half-cell is the reduction half-cell. ________ 10. A positive value for a standard reduction potential means hydrogen ions have a ...
... ________ 8. The half-cell that has a greater tendency to acquire electrons will be the one in which oxidation occurs. ________ 9. In an electrochemical cell, the hydrogen half-cell is the reduction half-cell. ________ 10. A positive value for a standard reduction potential means hydrogen ions have a ...
Chlorine
... As a result, diaphragm methods produce alkali that is quite dilute ( about 12 % ) and of lower purity than do mercury cell methods. But diaphragm cells are not burdened with the problem of preventing mercury discharge into the environment. They also operate at a lower voltage, resulting in an energy ...
... As a result, diaphragm methods produce alkali that is quite dilute ( about 12 % ) and of lower purity than do mercury cell methods. But diaphragm cells are not burdened with the problem of preventing mercury discharge into the environment. They also operate at a lower voltage, resulting in an energy ...
Formic acid oxidation reaction on a PdxNiy bimetallic nanoparticle
... formic acid-based fuel cells have been paid much attention essentially due to its unique advantages such as nontoxicity, inflammability at room temperature, higher theoretical open circuit potential, lower crossover through the membrane and the easy fuel storage and transport [2]. Thus, developing n ...
... formic acid-based fuel cells have been paid much attention essentially due to its unique advantages such as nontoxicity, inflammability at room temperature, higher theoretical open circuit potential, lower crossover through the membrane and the easy fuel storage and transport [2]. Thus, developing n ...
Answers - logo Pre-U Chemistry Textbook
... Two electrons would have to go into an antibonding molecular orbital. This is energetically unfavourable because the increase in energy of the antibonding orbital is greater than the decrease in energy of the bonding orbital. Helium molecules do not form because they would be at higher energy than t ...
... Two electrons would have to go into an antibonding molecular orbital. This is energetically unfavourable because the increase in energy of the antibonding orbital is greater than the decrease in energy of the bonding orbital. Helium molecules do not form because they would be at higher energy than t ...
Solids Chemistry XII - The Gurukul Institute
... Suppose a solid solution is formed between two substances, one of whose particles are very large and the other whose particles are very small, what kind of solid solution in this likely to be? Define ‘mole fraction of a component’ in a solution. Write an expression for mole fraction of a component ‘ ...
... Suppose a solid solution is formed between two substances, one of whose particles are very large and the other whose particles are very small, what kind of solid solution in this likely to be? Define ‘mole fraction of a component’ in a solution. Write an expression for mole fraction of a component ‘ ...
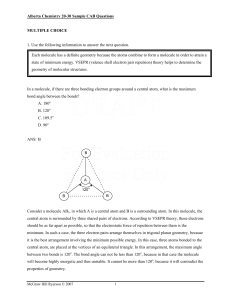
Alberta Chemistry 20-30 Sample CAB Questions - McGraw
... Consider a molecule AB3, in which A is a central atom and B is a surrounding atom. In this molecule, the central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between ...
... Consider a molecule AB3, in which A is a central atom and B is a surrounding atom. In this molecule, the central atom is surrounded by three shared pairs of electrons. According to VSEPR theory, these electrons should be as far apart as possible, so that the electrostatic force of repulsion between ...
Modern inorganic chemistry
... The welcome changes in GCE Advanced level syllabuses during the last few years have prompted the writing of this new Inorganic Chemistry which is intended to replace the book by Wood and Holliday. This new book, like its predecessor, should also be of value in first-year tertiary level chemistry cou ...
... The welcome changes in GCE Advanced level syllabuses during the last few years have prompted the writing of this new Inorganic Chemistry which is intended to replace the book by Wood and Holliday. This new book, like its predecessor, should also be of value in first-year tertiary level chemistry cou ...
File
... d Anions are negatively charged ions because they have more electrons than protons. Element E (Br–) is the only anion. e For atoms and ions to have the same electronic configuration, they must have the same number of electrons. Element A (K+) and F (Ar) both have 18 electrons, arranged 2,8,8 ...
... d Anions are negatively charged ions because they have more electrons than protons. Element E (Br–) is the only anion. e For atoms and ions to have the same electronic configuration, they must have the same number of electrons. Element A (K+) and F (Ar) both have 18 electrons, arranged 2,8,8 ...
KHARKOV STATE MEDICAL UNIVERSITY
... that have been created artificially are counted) are termed the Actinide elements. Lanthanide and Actinide form two rows of 14 elements at the bottom of the table. 2. Biogenic elements: general aspects Living organisms, like all other matter on earth, are composed of atoms of the naturally occurring ...
... that have been created artificially are counted) are termed the Actinide elements. Lanthanide and Actinide form two rows of 14 elements at the bottom of the table. 2. Biogenic elements: general aspects Living organisms, like all other matter on earth, are composed of atoms of the naturally occurring ...
Atomic Structure
... The statements (i) “In filling a group of orbitals of equal energy it is energetically preferable to assign electrons to empty orbitals rather than pair them into a particular orbital. (ii) When two electrons are placed in two different orbitals, energy is lower if the espins are parallel” are valid ...
... The statements (i) “In filling a group of orbitals of equal energy it is energetically preferable to assign electrons to empty orbitals rather than pair them into a particular orbital. (ii) When two electrons are placed in two different orbitals, energy is lower if the espins are parallel” are valid ...
Lessons 9
... Thermochemistry is the study of energy changes involved in chemical reactions. These changes can be physical (i.e. melting ice,) chemical (rusting iron) or nuclear (nuclear fusion reactions in the Sun). All energy transformations are governed by the 1st Law of Thermodynamics, which states that the t ...
... Thermochemistry is the study of energy changes involved in chemical reactions. These changes can be physical (i.e. melting ice,) chemical (rusting iron) or nuclear (nuclear fusion reactions in the Sun). All energy transformations are governed by the 1st Law of Thermodynamics, which states that the t ...
Water splitting

Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in the United States. In photosynthesis, water splitting donates electrons to power the electron transport chain in photosystem II.