Chemistry I Exams and Keys 2014 Season
... rises over several minutes. Eventually, bubbles appear at the bottom of the beaker and rise to the top of the liquid. More bubbles begin to appear all over the volume of the water as it starts to boil. After boiling continues for several minutes, which statement or statements below is/are completely ...
... rises over several minutes. Eventually, bubbles appear at the bottom of the beaker and rise to the top of the liquid. More bubbles begin to appear all over the volume of the water as it starts to boil. After boiling continues for several minutes, which statement or statements below is/are completely ...
CHEM 13 NEWS EXAM 1998 - University of Waterloo
... Mg gains electrons more readily than Fe, keeping the Fe from oxidizing. ...
... Mg gains electrons more readily than Fe, keeping the Fe from oxidizing. ...
Exam 2 Review - Iowa State University
... d. F, Cl, Br, I = -1 in binary compounds with metals e. H = +1 (-1 in metallic hydrides) f. O = -2 4. The sum of oxidation numbers of all atoms in a compound equal the charge of that compound 1. Identify the oxidation number of each individual atom in the following equation. a. Which atom is being o ...
... d. F, Cl, Br, I = -1 in binary compounds with metals e. H = +1 (-1 in metallic hydrides) f. O = -2 4. The sum of oxidation numbers of all atoms in a compound equal the charge of that compound 1. Identify the oxidation number of each individual atom in the following equation. a. Which atom is being o ...
apes review - Pace Ap Environmental Science
... 88. Municipal solid waste is mostly: paper and most is landfilled 89. True cost / External costs: harmful environmental side effects that are not reflected in a products price ...
... 88. Municipal solid waste is mostly: paper and most is landfilled 89. True cost / External costs: harmful environmental side effects that are not reflected in a products price ...
Chapter 23 (Section 3) Pregnancy, Birth, and Childhood
... *b. elements can _____ be ___________ down into other substances by any ______________ means c. some ___________ exists in elemental form [(e.g.) gold [___] = not chemically ____________)] *d. ____________ individually or combined form everything in the universe including __________ *1. Human body’s ...
... *b. elements can _____ be ___________ down into other substances by any ______________ means c. some ___________ exists in elemental form [(e.g.) gold [___] = not chemically ____________)] *d. ____________ individually or combined form everything in the universe including __________ *1. Human body’s ...
Chemical Basis of Life
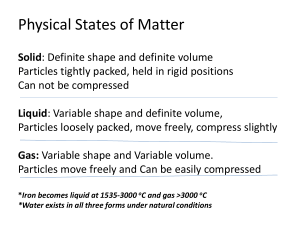
... Any substance that has mass and takes up space Composed of 1 or more elements Found in 1 of 3 states Gas – no definite shape or volume Liquid – shape conforms to container ...
... Any substance that has mass and takes up space Composed of 1 or more elements Found in 1 of 3 states Gas – no definite shape or volume Liquid – shape conforms to container ...
Chemical Questions
... • Strategies to prevent this: – Use heaters to heat orchard air. – Spray warm water on tree trunks; as water cools, heat is released to heat orchard air. – Spray water on fruit if T < 0 oC so liquid water ...
... • Strategies to prevent this: – Use heaters to heat orchard air. – Spray warm water on tree trunks; as water cools, heat is released to heat orchard air. – Spray water on fruit if T < 0 oC so liquid water ...
Lecture 11 - AP Chem Solutions
... compounds containing group 1A ions, nitrate, and ammonium are always soluble. 2) A potassium hydroxide solution is mixed with a solution of zinc nitrate. The potassium ion is always soluble as it is a Group 1A element. Nitrate is also soluble with everything. Thus, K+ and NO3- must be spectator ions ...
... compounds containing group 1A ions, nitrate, and ammonium are always soluble. 2) A potassium hydroxide solution is mixed with a solution of zinc nitrate. The potassium ion is always soluble as it is a Group 1A element. Nitrate is also soluble with everything. Thus, K+ and NO3- must be spectator ions ...
Document
... 88. Municpal solid waste is mostly: paper and most is landfilled 89. True cost / External costs: harmful environmental side effects that are not reflected in a products price 90. Sanitary landfill problems and solutions: (leachate, liner with collection system) (methane gas, collect gas and burn) (v ...
... 88. Municpal solid waste is mostly: paper and most is landfilled 89. True cost / External costs: harmful environmental side effects that are not reflected in a products price 90. Sanitary landfill problems and solutions: (leachate, liner with collection system) (methane gas, collect gas and burn) (v ...
CHEMISTRY-A SCIENCE FOR 21st Century
... *Changes associate with chemical properties result from the interaction of a substance with one or more other substances *Sometimes the presence of energy (heat, light) and pressure also triggers the change. (ex: decomposition of Hydrogen peroxide into water and oxygen) ...
... *Changes associate with chemical properties result from the interaction of a substance with one or more other substances *Sometimes the presence of energy (heat, light) and pressure also triggers the change. (ex: decomposition of Hydrogen peroxide into water and oxygen) ...
Redox Reactions Test Review
... 2. What is the common oxidation number for oxygen? What is the oxidation number for oxygen in H2O2 (the exception)? ...
... 2. What is the common oxidation number for oxygen? What is the oxidation number for oxygen in H2O2 (the exception)? ...
CHAPTER 2 THE CHEMISTRY OF LIFE 2.1 Chemical Elements
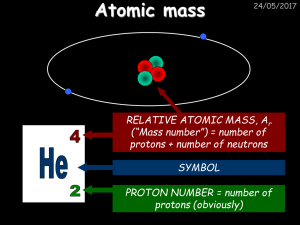
... Matter is defined as anything that has mass and takes up space. Both living and nonliving matter is composed of elements, the simplest forms of matter that cannot be broken down to simpler substances with different chemical or physical properties. Six of the elements that occur in nature—carbon, hyd ...
... Matter is defined as anything that has mass and takes up space. Both living and nonliving matter is composed of elements, the simplest forms of matter that cannot be broken down to simpler substances with different chemical or physical properties. Six of the elements that occur in nature—carbon, hyd ...
8492_Chemichal Weapons Production Indicators
... Phosgene is used as an intermediate in the manufacture of many organic chemicals. The largest amount (approximately 80% of world production) is used to produce toluene diisocyanate and other isocyanates. ...
... Phosgene is used as an intermediate in the manufacture of many organic chemicals. The largest amount (approximately 80% of world production) is used to produce toluene diisocyanate and other isocyanates. ...
Science 9
... In her notebook, the student recorded the final mass of the products, it was 140 g. Did this reaction conserve mass? Explain your answer. ...
... In her notebook, the student recorded the final mass of the products, it was 140 g. Did this reaction conserve mass? Explain your answer. ...
2nd nine weeks benchmark review homework
... substances. b- It dissolves only solid substances. c- It dissolves the greatest number of substances. d- It dissolves substances faster than all other solvents. The left side of a chemical equation are called the- ...
... substances. b- It dissolves only solid substances. c- It dissolves the greatest number of substances. d- It dissolves substances faster than all other solvents. The left side of a chemical equation are called the- ...
Answers to 2017 Chemistry Exam Review Compounds and
... (Notice that water is also amphoteric – acting as a base in the first example and an acid in the second.) 59. Kw = [H3O+][OH-] = 1.0 x 10-14 This is a very small number, meaning water rarely selfionizes. 60. [H+] = 1.0 x 10-14 / [OH-] or [OH-] = 1.0 x 10-14 / [H+] 61. pH = the negative power of ten ...
... (Notice that water is also amphoteric – acting as a base in the first example and an acid in the second.) 59. Kw = [H3O+][OH-] = 1.0 x 10-14 This is a very small number, meaning water rarely selfionizes. 60. [H+] = 1.0 x 10-14 / [OH-] or [OH-] = 1.0 x 10-14 / [H+] 61. pH = the negative power of ten ...
Water splitting

Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in the United States. In photosynthesis, water splitting donates electrons to power the electron transport chain in photosystem II.