Balancing Chemical Equations Activity by Liz LaRosa www
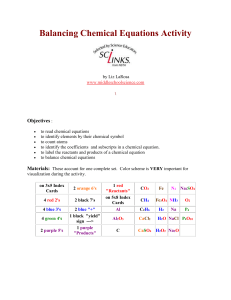
... Print activity cards on card stock instead of making index cards for quicker set up. The color coding is very important for visualization. It is easier and quicker to locate the elements that you are trying to balance. If everything is in black ink, its harder to distinguish the equation contents. I ...
... Print activity cards on card stock instead of making index cards for quicker set up. The color coding is very important for visualization. It is easier and quicker to locate the elements that you are trying to balance. If everything is in black ink, its harder to distinguish the equation contents. I ...
Answers for Review Questions Exam 3
... 10. Electrolysis is used as a source of elements from their ions. Ex. Na from Molten NaCl, Cl2 from a NaCl solution. 11. 0.1663 A current is needed. 12. First 2.47 Volts should be 2.47 Amperes. That gives 4.100g of Fe deposited. 13. Corrosion is the loss of metals to a solution of some form. The pro ...
... 10. Electrolysis is used as a source of elements from their ions. Ex. Na from Molten NaCl, Cl2 from a NaCl solution. 11. 0.1663 A current is needed. 12. First 2.47 Volts should be 2.47 Amperes. That gives 4.100g of Fe deposited. 13. Corrosion is the loss of metals to a solution of some form. The pro ...
File
... Physical or chemical change? The rain turned to snow… Marty broke a class on the bathroom floor… I burned my bagel! I fried eggs for breakfast… I mixed baking soda and vinegar for science ...
... Physical or chemical change? The rain turned to snow… Marty broke a class on the bathroom floor… I burned my bagel! I fried eggs for breakfast… I mixed baking soda and vinegar for science ...
IPC Final Exam Review
... PHYSICAL CHANGE VS CHEMICAL CHANGE Mark P for a physical change and C for a chemical change. ______1. Dew forms on the grass when the temperature drops at night. ______2. A bolt of lightening causes oxygen to change into ozone. ______3. Acid rain errodes away the face of a statue. ______4. Separati ...
... PHYSICAL CHANGE VS CHEMICAL CHANGE Mark P for a physical change and C for a chemical change. ______1. Dew forms on the grass when the temperature drops at night. ______2. A bolt of lightening causes oxygen to change into ozone. ______3. Acid rain errodes away the face of a statue. ______4. Separati ...
Chemistry IGCSE Revision PDF File
... If a metal is more reactive than hydrogen its ions stay in solution and hydrogen bubbles off ...
... If a metal is more reactive than hydrogen its ions stay in solution and hydrogen bubbles off ...
Chapter 8
... amount of energy in the form of light and heat. Reactive elements combine with oxygen ...
... amount of energy in the form of light and heat. Reactive elements combine with oxygen ...
File
... • 1.2 Questions: Pg. 23-24 #1-5 • Diagrams of Atoms Summative Assignment–Due Monday, February 8th. ...
... • 1.2 Questions: Pg. 23-24 #1-5 • Diagrams of Atoms Summative Assignment–Due Monday, February 8th. ...
Class Activity
... crystalline form, boiling point, freezing point, and vapor pressure. Chemical Change: Chemical change is associated with change that results in a new substance with different properties. A chemical reaction is normally not easily reversed. For example, burning a piece of magnesium in oxygen produces ...
... crystalline form, boiling point, freezing point, and vapor pressure. Chemical Change: Chemical change is associated with change that results in a new substance with different properties. A chemical reaction is normally not easily reversed. For example, burning a piece of magnesium in oxygen produces ...
CHM2045 Final Exam Review, Spring 2017
... container is 4 atm. Assuming the reaction proceeds to completion, what will be the pressure exerted on the vessel after the reaction takes place? ...
... container is 4 atm. Assuming the reaction proceeds to completion, what will be the pressure exerted on the vessel after the reaction takes place? ...
Reactions
... • Usually end with the suffix “-ase” and its name comes from the substrate • Have optimal temperatures and pH to maintain normal functioning • Enzymes lower the activation energy of the reaction • Activation energy – the minimum amount of energy needed to cause a chemical reaction to occur • Without ...
... • Usually end with the suffix “-ase” and its name comes from the substrate • Have optimal temperatures and pH to maintain normal functioning • Enzymes lower the activation energy of the reaction • Activation energy – the minimum amount of energy needed to cause a chemical reaction to occur • Without ...
The bombardier beetle uses an explosive discharge as a defensive
... 2. A hot air balloon is being inflated to its full extent by heating the air inside it. In the final stages of this process, the volume of the balloon changes from 3.5 x 106 L to 4.50 x 106 L by the addition of 160 MJ of energy as heat. Assuming that the balloon expands against a constant pressure o ...
... 2. A hot air balloon is being inflated to its full extent by heating the air inside it. In the final stages of this process, the volume of the balloon changes from 3.5 x 106 L to 4.50 x 106 L by the addition of 160 MJ of energy as heat. Assuming that the balloon expands against a constant pressure o ...
CfE Higher Chemistry Homework 3.5
... In which of the following reactions would an increase in pressure cause the equilibrium position to move to the left? ...
... In which of the following reactions would an increase in pressure cause the equilibrium position to move to the left? ...
Chapter 2 Study Guides
... 9. In an endothermic reaction, the products have a __________________ bond energy than the reactants. Overall, energy is __________________. ...
... 9. In an endothermic reaction, the products have a __________________ bond energy than the reactants. Overall, energy is __________________. ...
General CHemistry Unit 2 Homework Notes
... The only way to form a compound from elements is by a chemical reaction. Example: Hydrogen gas and oxygen gas react to synthesize water. 2H2 + O2 2H2O The only way to separate a compound into its elements is by a chemical reaction that breaks the chemical bonds, forming new substances. (Example: w ...
... The only way to form a compound from elements is by a chemical reaction. Example: Hydrogen gas and oxygen gas react to synthesize water. 2H2 + O2 2H2O The only way to separate a compound into its elements is by a chemical reaction that breaks the chemical bonds, forming new substances. (Example: w ...
Too Hot to Handle Lab
... (endothermic), and where heat is lost (exothermic). Background: A Chemical reaction in which energy is released is an exothermic reaction. The word exothermic comes from the root – “thermic”, which refers to heat, and the prefix – “exo” which means out of. Heat comes out of, or is released from, a r ...
... (endothermic), and where heat is lost (exothermic). Background: A Chemical reaction in which energy is released is an exothermic reaction. The word exothermic comes from the root – “thermic”, which refers to heat, and the prefix – “exo” which means out of. Heat comes out of, or is released from, a r ...
Water splitting

Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in the United States. In photosynthesis, water splitting donates electrons to power the electron transport chain in photosystem II.























