Chapter 13 Notes
... A single replacement reaction that involves oxidation and reduction occurs in the steel-making process. ...
... A single replacement reaction that involves oxidation and reduction occurs in the steel-making process. ...
Introduction to Chemical Equations
... Matter is being rearranged, but NO mass is lost. If you were to collect all of the products and measure their mass, it would be equal to the original mass of the wood. ...
... Matter is being rearranged, but NO mass is lost. If you were to collect all of the products and measure their mass, it would be equal to the original mass of the wood. ...
C1a - Mr Corfe
... EXOTHERMIC – gives out heat ENDOTHERMIC – take in heat from it surrounding THERMAL DECOMPOSITION – is a chemical reaction where a single compound breaks up into two or more simpler compounds or elements when heated DEHYDRATION – chemical reaction that involves the loss of water from the reacting mol ...
... EXOTHERMIC – gives out heat ENDOTHERMIC – take in heat from it surrounding THERMAL DECOMPOSITION – is a chemical reaction where a single compound breaks up into two or more simpler compounds or elements when heated DEHYDRATION – chemical reaction that involves the loss of water from the reacting mol ...
IntroRedoxDCIAns
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
Introduction to Oxidation Reduction
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
... The first three reactions show an element, in this case oxygen, converted to the combined form of oxygen in a compound. An element was converted to a compound in the reactions. In the fourth reaction, a compound decomposed into its elements. General statement-the reactions show the disappearance or ...
Solutions - Seattle Central
... Test for Simple Carbohydrates: Benedict’s Benedict's solution is a chemical indicator for simple sugars such as glucose: C6H12O6. Unlike some other indicators, Benedict’s solution does not work at room temperature - it must be heated first Details: ...
... Test for Simple Carbohydrates: Benedict’s Benedict's solution is a chemical indicator for simple sugars such as glucose: C6H12O6. Unlike some other indicators, Benedict’s solution does not work at room temperature - it must be heated first Details: ...
File
... • Each group should have 4 people and 20 cards. Split the cards up so each person has 5. • On your own piece of paper, write out your 5 chemical equations and balance them. • Then, once all equations are balanced, look at the 20 as a group. You need to split the 20 cards up in to 5 different react ...
... • Each group should have 4 people and 20 cards. Split the cards up so each person has 5. • On your own piece of paper, write out your 5 chemical equations and balance them. • Then, once all equations are balanced, look at the 20 as a group. You need to split the 20 cards up in to 5 different react ...
Teacher Demo/Student Activity: Elephant`s Toothpaste
... rate of this reaction. Catalysts are substances that increase the rate of a reaction by lowering the activation energy of that reaction. Catalysts differ from reactants in that they are not consumed in the reaction. Manganese(IV) oxide, potassium iodide, sodium iodide, and yeast are examples of cata ...
... rate of this reaction. Catalysts are substances that increase the rate of a reaction by lowering the activation energy of that reaction. Catalysts differ from reactants in that they are not consumed in the reaction. Manganese(IV) oxide, potassium iodide, sodium iodide, and yeast are examples of cata ...
Exam practice answers 5
... to give the overall equation: 2MnO4–(aq) + 16H+(aq) + 10I–(aq) 2Mn2+ + 8H2O(l) + 5I2(aq) 4 (a) Hydrogen and oxygen are supplied to the fuel cell. If an acidic catalyst is used: At the positive electrode the fuel (hydrogen gas) is converted to hydrogen ions: H2(g) 2H+(aq) + 2e– At the negat ...
... to give the overall equation: 2MnO4–(aq) + 16H+(aq) + 10I–(aq) 2Mn2+ + 8H2O(l) + 5I2(aq) 4 (a) Hydrogen and oxygen are supplied to the fuel cell. If an acidic catalyst is used: At the positive electrode the fuel (hydrogen gas) is converted to hydrogen ions: H2(g) 2H+(aq) + 2e– At the negat ...
Unit 1 Exam Review
... attracts hydrogen atoms of another • Water’s strong cohesion allows nutrients and waste to be transported • Water absorbs heat with only small changes in its temperature, which stabilizes systems ...
... attracts hydrogen atoms of another • Water’s strong cohesion allows nutrients and waste to be transported • Water absorbs heat with only small changes in its temperature, which stabilizes systems ...
Elementary my dear Watson review
... product (fizz!) only when we placed a balloon on top of the Erlenmeyer Flask to catch the gas (CO2) that was produced. Otherwise, the mass after the reaction would have decreased because the produced CO2 could have escaped. ...
... product (fizz!) only when we placed a balloon on top of the Erlenmeyer Flask to catch the gas (CO2) that was produced. Otherwise, the mass after the reaction would have decreased because the produced CO2 could have escaped. ...
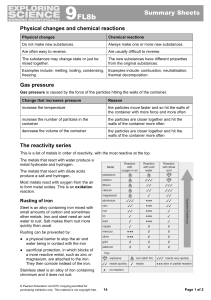
9F Reactivity - Parrs Wood High School
... This is a list of metals in order of reactivity, with the most reactive at the top. The metals that react with water produce a metal hydroxide and hydrogen. The metals that react with dilute acids produce a salt and hydrogen. Most metals react with oxygen from the air to form metal oxides. This is a ...
... This is a list of metals in order of reactivity, with the most reactive at the top. The metals that react with water produce a metal hydroxide and hydrogen. The metals that react with dilute acids produce a salt and hydrogen. Most metals react with oxygen from the air to form metal oxides. This is a ...
Hydrogen peroxide solution about 30% w/v AnalaR
... If local regulations permit, mop up with plenty of water and run to waste, diluting greatly with running water. Otherwise absorb on an inert absorbent, transfer to container and arrange removal by disposal company. Ventilate area to dispel residual vapour. For large spillages liquids should be conta ...
... If local regulations permit, mop up with plenty of water and run to waste, diluting greatly with running water. Otherwise absorb on an inert absorbent, transfer to container and arrange removal by disposal company. Ventilate area to dispel residual vapour. For large spillages liquids should be conta ...
The only sure evidence that a chemical reaction has occured is
... 13. Which reaction requires a continuous supply of energy in order to continue? ...
... 13. Which reaction requires a continuous supply of energy in order to continue? ...
Chemical Equations and Reactions
... – Some form of energy is given off by the reaction • Heat given off causes reaction mixture to feel hot • Examples-burning wood, dynamite explosion ...
... – Some form of energy is given off by the reaction • Heat given off causes reaction mixture to feel hot • Examples-burning wood, dynamite explosion ...
Water splitting

Water splitting is the general term for a chemical reaction in which water is separated into oxygen and hydrogen. Efficient and economical water splitting would be a key technology component of a hydrogen economy. Various techniques for water splitting have been issued in water splitting patents in the United States. In photosynthesis, water splitting donates electrons to power the electron transport chain in photosystem II.