01/27/2012 - The Medical University of South Carolina
... CDC - Centers for Disease Control and Prevention; an agency within the Public Health Service, Department of Health and Human Services. ...
... CDC - Centers for Disease Control and Prevention; an agency within the Public Health Service, Department of Health and Human Services. ...
PowerPoint_Chapter1
... Drug is tried in healthy human subjects Generic drug name assigned • Phase II and Phase III clinical trials Drug is tested in human having condition or disease the drug is meant to treat End of Phase III FDA approval sought and brand name assigned • Phase IV clinical trials Called postmark ...
... Drug is tried in healthy human subjects Generic drug name assigned • Phase II and Phase III clinical trials Drug is tested in human having condition or disease the drug is meant to treat End of Phase III FDA approval sought and brand name assigned • Phase IV clinical trials Called postmark ...
IN VITRO COMPLEXED NICARDIPINE HYDROCHLORIDE
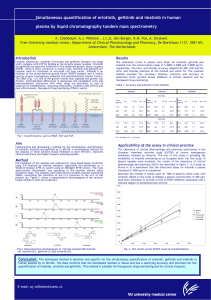
... Fig. 2: Dissolution profiles of various tablet formulations. ...
... Fig. 2: Dissolution profiles of various tablet formulations. ...
ROVI announces the marketing agreement of
... About Orexigen Therapeutics Orexigen Therapeutics, Inc. is a biopharmaceutical company focused on the treatment of obesity. Orexigen's first product, Contrave® (naltrexone HCl and bupropion HCl extended release), was approved in the United States in September 2014 and became the most prescribed bra ...
... About Orexigen Therapeutics Orexigen Therapeutics, Inc. is a biopharmaceutical company focused on the treatment of obesity. Orexigen's first product, Contrave® (naltrexone HCl and bupropion HCl extended release), was approved in the United States in September 2014 and became the most prescribed bra ...
Bruyere/Primrose Units - R. Halil, August 2015
... Lower requirements for license to sell, but claims of efficacy are more restricted Toxicity Lower quality control requirements Risk of drug interactions Cost – Billions spent per year Convenience – no Rx needed. More “natural”. ...
... Lower requirements for license to sell, but claims of efficacy are more restricted Toxicity Lower quality control requirements Risk of drug interactions Cost – Billions spent per year Convenience – no Rx needed. More “natural”. ...
generic - World Health Organization
... and comparative clinical trials. To exert an optimal therapeutic action, an active pharmaceutical ingredient should be delivered to its site(s) of action in an effective concentration for the desired period. To allow reliable prediction of the therapeutic effect, the performance of the pharmaceutica ...
... and comparative clinical trials. To exert an optimal therapeutic action, an active pharmaceutical ingredient should be delivered to its site(s) of action in an effective concentration for the desired period. To allow reliable prediction of the therapeutic effect, the performance of the pharmaceutica ...
Preview Sample 1
... Liquid medications do not need to go through the dissolution phase and may be immediately absorbed. Tablets and capsules must be dissolved. Enteric-coated tablets are designed to be absorbed at a slower rate. DIF: Cognitive Level: Application REF: 3 TOP: Nursing Process: Intervention/Implementation ...
... Liquid medications do not need to go through the dissolution phase and may be immediately absorbed. Tablets and capsules must be dissolved. Enteric-coated tablets are designed to be absorbed at a slower rate. DIF: Cognitive Level: Application REF: 3 TOP: Nursing Process: Intervention/Implementation ...
Mechanistic Pharmacokinetic Modeling for the Prediction of
... The passive diffusion component was parameterized as unbound distribution CL (CLint, u, pass) within the model. Active uptake was parameterized in the ...
... The passive diffusion component was parameterized as unbound distribution CL (CLint, u, pass) within the model. Active uptake was parameterized in the ...
BASS & ULLMAN, I?G.
... these Acts were carefully crafted by Congress to combat the illicit use of ephedrine while, at the same time, ensuring the continued availability of legitimate products OTC to consumers who have come to know and to depend on them. Recognizing significant law enforcement concerns with single entity p ...
... these Acts were carefully crafted by Congress to combat the illicit use of ephedrine while, at the same time, ensuring the continued availability of legitimate products OTC to consumers who have come to know and to depend on them. Recognizing significant law enforcement concerns with single entity p ...
Drug Use During Pregnancy and Lactation
... • Otherwise, in addition to the pregnancy category, information on teratogenicity, effects on reproduction, and when available, effects on later growth, development and functional maturation of the child should be included ...
... • Otherwise, in addition to the pregnancy category, information on teratogenicity, effects on reproduction, and when available, effects on later growth, development and functional maturation of the child should be included ...
Titel voorbeeld titel
... and gefitinib are selective inhibitors of the epidermal growth factor receptor (EGFR), which is often overactive in tumors cells. Erlotinib and gefitinib are common used for treatment of non-small cell lung cancer. Imatinib is an inhibitor of the platet-derived growth factor (PDGF)-receptor and is m ...
... and gefitinib are selective inhibitors of the epidermal growth factor receptor (EGFR), which is often overactive in tumors cells. Erlotinib and gefitinib are common used for treatment of non-small cell lung cancer. Imatinib is an inhibitor of the platet-derived growth factor (PDGF)-receptor and is m ...
Therapeutic Drug Monitoring of Antiepileptic Drugs in the 21st Century
... distinguished. There can be either an indication for systematic TDM on a regular basis, or a need for initial adjustment up to finding the right dose, or solely an indication for “on-need” control when confronted with clinical issues such as treatment resistance, side effects, or drug interactions. ...
... distinguished. There can be either an indication for systematic TDM on a regular basis, or a need for initial adjustment up to finding the right dose, or solely an indication for “on-need” control when confronted with clinical issues such as treatment resistance, side effects, or drug interactions. ...
Community/Ambulatory Care Edition December 2008 Vol.7, Issue 12
... or hazardous condition, please access the ISMP MERP on our website at: www.ismp.org. ...
... or hazardous condition, please access the ISMP MERP on our website at: www.ismp.org. ...
FORMULATION DESIGN AND OPTIMIZATION OF HERBAL GEL CONTAINING ALBIZIA Original Article
... Herbal medicine has become an item of global importance both medicinal and economical. Although usage of these herbal medicines has increased, their quality, safety and efficiency are serious concerns in industrialized and developing countries. Plants play a vital role in curing various ailments of ...
... Herbal medicine has become an item of global importance both medicinal and economical. Although usage of these herbal medicines has increased, their quality, safety and efficiency are serious concerns in industrialized and developing countries. Plants play a vital role in curing various ailments of ...
Introduction to Pharmacology 2 General Principles: Absorption
... be better absorbed. o Absorption of Drugs from Various Sites Oral or nasal mucosa- pH = 6-8; neutral drugs or weak bases are absorbed by-pass digestion Good blood supply and small surface area weak bases (cocaine) or neutral (nitro) can be absorbed ...
... be better absorbed. o Absorption of Drugs from Various Sites Oral or nasal mucosa- pH = 6-8; neutral drugs or weak bases are absorbed by-pass digestion Good blood supply and small surface area weak bases (cocaine) or neutral (nitro) can be absorbed ...
storage, stability and in-use shelf-life guidelines for non
... based on generalisations with regard to the dosage form of the medicine. As such there will always be exceptions to these rules and the use of professional judgement is still required. It is important that all medicines in bulk containers are dated on first opening so that such judgements can be mad ...
... based on generalisations with regard to the dosage form of the medicine. As such there will always be exceptions to these rules and the use of professional judgement is still required. It is important that all medicines in bulk containers are dated on first opening so that such judgements can be mad ...
Engineering Simulation Solutions for the Biomed Industry
... in the biomedical industry are transforming their leading-edge design concepts into innovative products and processes that work. Today, 97 of the top 100 industrial companies on the “FORTUNE Global 500” invest in engineering simulation as a key strategy to win in a globally competitive environment. ...
... in the biomedical industry are transforming their leading-edge design concepts into innovative products and processes that work. Today, 97 of the top 100 industrial companies on the “FORTUNE Global 500” invest in engineering simulation as a key strategy to win in a globally competitive environment. ...
Drug Facts Sheet Hydrocodone
... Hydrocodone is the most frequently prescribed opioid in the United States and is associated with more drug abuse and diversion than any other licit or illicit opioid. It is an orally active agent most frequently prescribed for the treatment of moderate to moderately severe pain. It’s analgesic poten ...
... Hydrocodone is the most frequently prescribed opioid in the United States and is associated with more drug abuse and diversion than any other licit or illicit opioid. It is an orally active agent most frequently prescribed for the treatment of moderate to moderately severe pain. It’s analgesic poten ...
High Cost Drugs policy - Province of British Columbia
... A discount paid or credited by a supplier for prompt payment of invoices is not included in the calculation of AAC. (The PharmaCare-recognized discount is usually no more than two per cent.) ...
... A discount paid or credited by a supplier for prompt payment of invoices is not included in the calculation of AAC. (The PharmaCare-recognized discount is usually no more than two per cent.) ...
Drug, substance that affects the function of living cells
... Distribution is the transport of a drug from the bloodstream to tissue sites where it will be effective, as well as to sites where the drug may be stored, metabolized, or eliminated from the body. Once a drug reaches its intended destination, the drug molecules move from blood through cellular barri ...
... Distribution is the transport of a drug from the bloodstream to tissue sites where it will be effective, as well as to sites where the drug may be stored, metabolized, or eliminated from the body. Once a drug reaches its intended destination, the drug molecules move from blood through cellular barri ...