In cattle - Veterinary Medicines Directorate
... the liver is the target tissue with the highest residue levels recovered. All other tissues showed lower residues: fat> kidney>muscle. The injection site had residues shortly after treatment but by day 28 the average residues were negligible. After administration of tritium-labelled ivermectin, faec ...
... the liver is the target tissue with the highest residue levels recovered. All other tissues showed lower residues: fat> kidney>muscle. The injection site had residues shortly after treatment but by day 28 the average residues were negligible. After administration of tritium-labelled ivermectin, faec ...
Basics Pharmacology Review Part 2 - Dr. Halil
... So? • So…. • If equivalent LDL lowering with non-statin drugs have no effect on morbidity or mortality then LDL may only be a surrogate marker of the pleiotrophic effects of statins. ...
... So? • So…. • If equivalent LDL lowering with non-statin drugs have no effect on morbidity or mortality then LDL may only be a surrogate marker of the pleiotrophic effects of statins. ...
Metabolic Disorders - Pipeline Review, H1 2013 Brochure
... and featured press releases from company/university sites and industry-specific third party sources, put together by Global Markets Direct’s team. Note*: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease. Scope - A snap ...
... and featured press releases from company/university sites and industry-specific third party sources, put together by Global Markets Direct’s team. Note*: Certain sections in the report may be removed or altered based on the availability and relevance of data for the indicated disease. Scope - A snap ...
Document
... – Pathogen can acquire resistance to more than one drug – Common when R-plasmids exchanged – Develop in hospitals and nursing homes – Constant use of drugs eliminates sensitive cells ...
... – Pathogen can acquire resistance to more than one drug – Common when R-plasmids exchanged – Develop in hospitals and nursing homes – Constant use of drugs eliminates sensitive cells ...
Afrezza®: Inhaled insulin approved by the FDA
... 1 and 2 diabetics will still need an injection of longer acting insulin to maintain a basal level for a 24-hour period http://www.nature.com/nbt/journal/v25/n12/full/nbt1207-1331.html ...
... 1 and 2 diabetics will still need an injection of longer acting insulin to maintain a basal level for a 24-hour period http://www.nature.com/nbt/journal/v25/n12/full/nbt1207-1331.html ...
annex iv: biowaiver request for additional strengths
... Biowaiver: The term biowaiver is applied to a regulatory drug approval process when the dossier (application) is approved based on evidence of equivalence other than through in vivo equivalence testing. Comparator product: is a pharmaceutical product with which the generic product is intended to be ...
... Biowaiver: The term biowaiver is applied to a regulatory drug approval process when the dossier (application) is approved based on evidence of equivalence other than through in vivo equivalence testing. Comparator product: is a pharmaceutical product with which the generic product is intended to be ...
CHR1sPPHER WEISBERG, EA.
... However, the severity of symptomshas been mainly m ild and transient by nature. The eventsgrouped as “metabolic/nutritional” show no sign of causality by CLA or placebo. The occurrenceof disordersof the endocrine systemin females is confined to four registrations of elevated TSH and four casesof “hy ...
... However, the severity of symptomshas been mainly m ild and transient by nature. The eventsgrouped as “metabolic/nutritional” show no sign of causality by CLA or placebo. The occurrenceof disordersof the endocrine systemin females is confined to four registrations of elevated TSH and four casesof “hy ...
Design and Evaluation of Niosomal Gel Delivery Systems for Topical
... was entrapped within the lipid bilayers formed by this technique with high efficiency. A significant amount of non-ionic surfactant (span 40) in the proniosomal formulation was needed to enhance estradiol permeation across skin. The findings suggest that inclusion of surfactants and lecithin in vesi ...
... was entrapped within the lipid bilayers formed by this technique with high efficiency. A significant amount of non-ionic surfactant (span 40) in the proniosomal formulation was needed to enhance estradiol permeation across skin. The findings suggest that inclusion of surfactants and lecithin in vesi ...
Lyrica (Epilepsy) - Forecast and Market Analysis to 2022 Brochure
... Other key drugs include older generation AEDs such as Pfizer’s Dilantin, Abbott’s Depakote, and Novartis’ Tegretol and Trileptal which still have significant usage due to their longevity in the market. However, the AED dominance landscape will continue to shift towards newer generation drugs particu ...
... Other key drugs include older generation AEDs such as Pfizer’s Dilantin, Abbott’s Depakote, and Novartis’ Tegretol and Trileptal which still have significant usage due to their longevity in the market. However, the AED dominance landscape will continue to shift towards newer generation drugs particu ...
Stability Testing of Pharmaceutical Products
... bracket tables for prediction of shelf life of the products but these methods are not official either in ICH or FDA. The Q rule states that a product degradation rate decreases by a constant factor Q10 when the storage temperature is decreased by 10°C. The value of Q10 is typically set at 2, 3 or 4 ...
... bracket tables for prediction of shelf life of the products but these methods are not official either in ICH or FDA. The Q rule states that a product degradation rate decreases by a constant factor Q10 when the storage temperature is decreased by 10°C. The value of Q10 is typically set at 2, 3 or 4 ...
questions and answers about mesotherapy
... itchiness for 2-3 hours after their Mesotherapy. This can be controlled with an anti-histamine like Claratin. Loose fitting clothing and avoidance of vigorous exercise for 1 day after your Mesotherapy is advised. You may feel small lumps under your skin that are not painful or itchy after Mesotherap ...
... itchiness for 2-3 hours after their Mesotherapy. This can be controlled with an anti-histamine like Claratin. Loose fitting clothing and avoidance of vigorous exercise for 1 day after your Mesotherapy is advised. You may feel small lumps under your skin that are not painful or itchy after Mesotherap ...
Welcome to Week 3 Chapter 6 - Blood and Drug Transport 6.1
... Learning Goals: To understand the fluids of the body into which a drug can be transported. Drug concentrations are measured in plasma and reported as Cp. Drugs, however, certainly travel in other fluids in the body. Below are descriptions of the most relevant fluids for drugs in a 70‐kg patient ...
... Learning Goals: To understand the fluids of the body into which a drug can be transported. Drug concentrations are measured in plasma and reported as Cp. Drugs, however, certainly travel in other fluids in the body. Below are descriptions of the most relevant fluids for drugs in a 70‐kg patient ...
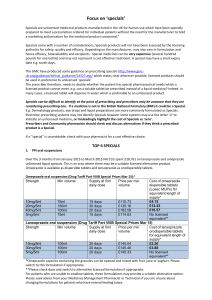
The Top 6 June specials 2015
... prepared to meet a prescription ordered for individual patients without the need for the manufacturer to hold a marketing authorisation for the medicinal product concerned.” Specials come with a number of considerations; Specials products will not have been assessed by the licensing authority for sa ...
... prepared to meet a prescription ordered for individual patients without the need for the manufacturer to hold a marketing authorisation for the medicinal product concerned.” Specials come with a number of considerations; Specials products will not have been assessed by the licensing authority for sa ...
Nexium News: the “Purple Pill” Goes Over-The
... Proton Pump Inhibitors such as Nexium are seen in workers’ compensation co-prescribed with nonsteroidal anti-inflammatory drugs (NSAIDs: naproxen and ibuprofen are examples) in certain patients considered at high risk for gastrointestinal ulcers. Since ALL PPIs are considered equally effective with ...
... Proton Pump Inhibitors such as Nexium are seen in workers’ compensation co-prescribed with nonsteroidal anti-inflammatory drugs (NSAIDs: naproxen and ibuprofen are examples) in certain patients considered at high risk for gastrointestinal ulcers. Since ALL PPIs are considered equally effective with ...
Average, population and individual bioequivalence
... 1902, it established the need for annual licensing of companies that manufactured and sold vaccines and serum derivatives. From that time on, labels for such products were required to contain the manufacturer’s name and licence number, and production was to be supervised by qualified professionals.1 ...
... 1902, it established the need for annual licensing of companies that manufactured and sold vaccines and serum derivatives. From that time on, labels for such products were required to contain the manufacturer’s name and licence number, and production was to be supervised by qualified professionals.1 ...
Bupropion/naltrexone fixed-dose combination for
... among others (8). A recent study has shown a direct association between obesity and all cause mortality (9). Accordingly, it is imperative to search for drugs that target the causal factor and not only its consequences. ...
... among others (8). A recent study has shown a direct association between obesity and all cause mortality (9). Accordingly, it is imperative to search for drugs that target the causal factor and not only its consequences. ...
GlycoGenesys, Inc. $1.10 BUY
... Compound Annual Growth Rates from 2009 to 2014...................................................50 Price Targets for LFY+2 for 3 Scenarios vs. Long Term Growth Rate.........................50 Price Targets Based on Future Events....................................................................... ...
... Compound Annual Growth Rates from 2009 to 2014...................................................50 Price Targets for LFY+2 for 3 Scenarios vs. Long Term Growth Rate.........................50 Price Targets Based on Future Events....................................................................... ...
168767_DrMaddin - Skin Therapy Letter
... Malathion lotion should be applied to dry hair, and then allowed to dry on the scalp. After several hours, the hair can be combed to remove nits and lice. Success rates of > 90% have been reported3. Ovide Lotion (Medicis), composed of 0.5% malathion in 78% isopropanol, was recently approved by the U ...
... Malathion lotion should be applied to dry hair, and then allowed to dry on the scalp. After several hours, the hair can be combed to remove nits and lice. Success rates of > 90% have been reported3. Ovide Lotion (Medicis), composed of 0.5% malathion in 78% isopropanol, was recently approved by the U ...
Study protocol In English (download here)
... Prescribing drugs safely and effectively is a fundamental skill that medical graduates must acquire, because after graduation they will prescribe drugs on a daily basis, often with minimal supervision. Effective undergraduate education in clinical pharmacology and therapeutics (CPT) is therefore ess ...
... Prescribing drugs safely and effectively is a fundamental skill that medical graduates must acquire, because after graduation they will prescribe drugs on a daily basis, often with minimal supervision. Effective undergraduate education in clinical pharmacology and therapeutics (CPT) is therefore ess ...