Chapter 32 Nuclear Physics
... The nucleus of an atom is incredibly small. The diameter of the nucleus is about 100,000 times smaller than the diameter of the atom. Although small, the nucleus contains more than 99.9% of the mass of an atom, because each of its constitutes (protons and neutrons) is about 1800 times more massive t ...
... The nucleus of an atom is incredibly small. The diameter of the nucleus is about 100,000 times smaller than the diameter of the atom. Although small, the nucleus contains more than 99.9% of the mass of an atom, because each of its constitutes (protons and neutrons) is about 1800 times more massive t ...
Nuclear For Forensics
... When the fission bomb is detonated, gamma rays and Xrays emitted first compress the fusion fuel, then heat it to thermonuclear temperatures ...
... When the fission bomb is detonated, gamma rays and Xrays emitted first compress the fusion fuel, then heat it to thermonuclear temperatures ...
Nuclear Chemistry
... Capture • If too many protons are present, another decay may occur where an inner shell electron is captured by the nucleus and combined with a proton to form a neutron; called electron capture. ...
... Capture • If too many protons are present, another decay may occur where an inner shell electron is captured by the nucleus and combined with a proton to form a neutron; called electron capture. ...
Chapter 21 Nuclear Chemistry - Ocean County Vocational
... Capture • If too many protons are present, another decay may occur where an inner shell electron is captured by the nucleus and combined with a proton to form a neutron; called electron capture. ...
... Capture • If too many protons are present, another decay may occur where an inner shell electron is captured by the nucleus and combined with a proton to form a neutron; called electron capture. ...
Energy Basics
... Energy - Ability to do work!! Work – application of force over distance (joules) Power – rate of energy flow of the rate of work done; ...
... Energy - Ability to do work!! Work – application of force over distance (joules) Power – rate of energy flow of the rate of work done; ...
A Conceptual Introduction to Chemistry, First Edition
... Critical mass – The smallest amount of fissionable material necessary to support a continuing chain reaction ...
... Critical mass – The smallest amount of fissionable material necessary to support a continuing chain reaction ...
radioactivity-ppt
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
Radioactivity
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
... unstable atomic nucleus loses energy by emitting radiation in the form of particles or electromagnetic waves. There are numerous types of radioactive decay. The ...
Radioisotopes
... (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number o ...
... (Nuclides) of the same chemical element, each having a different atomic mass (mass number) • Isotopes of an element have nuclei with the same number of protons (the same atomic number) but different numbers of neutrons. • Therefore, isotopes have different mass numbers, which give the total number o ...
Chapter 25
... isotopic symbols and apply the law of matter conservation. Let’s write the symbols for these particles. ...
... isotopic symbols and apply the law of matter conservation. Let’s write the symbols for these particles. ...
Nuclear Chemistry PowerPoint
... familiar with them as stimulated reactions. There are two such types of nuclear reactions: ...
... familiar with them as stimulated reactions. There are two such types of nuclear reactions: ...
Stable Vs Unstable Isotopes
... When an atom loses an alpha particle: its atomic number decreases by 2 units and its mass number decreases by 4 units neither its atomic number nor its mass number changes its mass number decreases by 1 unit but its atomic number remains unchanged its atomic number increases by 1 unit but its mass n ...
... When an atom loses an alpha particle: its atomic number decreases by 2 units and its mass number decreases by 4 units neither its atomic number nor its mass number changes its mass number decreases by 1 unit but its atomic number remains unchanged its atomic number increases by 1 unit but its mass n ...
Glossary of Technical Terms - Institute for Energy and Environmental
... as hydrogen or lithium gives a large relsease of energy. fission product Any isotope created by the fission of a heavy element. Fission products are usually radioactive. fission The splitting of the nucleus of an element into fragments. Heavy elements such as uranium or plutonium release energy when ...
... as hydrogen or lithium gives a large relsease of energy. fission product Any isotope created by the fission of a heavy element. Fission products are usually radioactive. fission The splitting of the nucleus of an element into fragments. Heavy elements such as uranium or plutonium release energy when ...
Unit 7 Review
... The uranium-235 nucleus is stationary at the time that the fission reaction occurs. In this fission reaction, 198 MeV of energy is released. Of this total energy, 102 MeV and 65 MeV are the kinetic energies of the strontium-90 and xenon-142 nuclei respectively. (b)(i) Suggest what has happened to t ...
... The uranium-235 nucleus is stationary at the time that the fission reaction occurs. In this fission reaction, 198 MeV of energy is released. Of this total energy, 102 MeV and 65 MeV are the kinetic energies of the strontium-90 and xenon-142 nuclei respectively. (b)(i) Suggest what has happened to t ...
nuclear reactions
... This is not the only possible reaction: a variety of daughter isotopes are produced (As, Br, Sr, Zn, and Zr), some of which are stable, but most of which are radioactive themselves (e.g. as -, + or emitters). These reaction can release 1, 2 or 3 neutrons, and on average 235U fission releases 2 n ...
... This is not the only possible reaction: a variety of daughter isotopes are produced (As, Br, Sr, Zn, and Zr), some of which are stable, but most of which are radioactive themselves (e.g. as -, + or emitters). These reaction can release 1, 2 or 3 neutrons, and on average 235U fission releases 2 n ...
Nuclear Chemistry
... First Isotope (bombarding particle, ejected particle) Final Isotope Notation for Nitrogen-14 bombarded with a particle. ...
... First Isotope (bombarding particle, ejected particle) Final Isotope Notation for Nitrogen-14 bombarded with a particle. ...
have shown no evidence
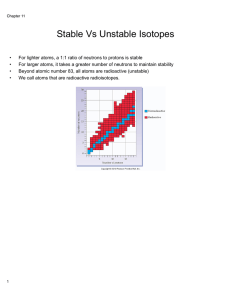
... • Stability is favoured by even numbers of protons and neutrons • Not usually equal numbers • Plotting neutron number (A) against proton number (Z) for all known nuclei, shows area of stability • For very light elements N ≈ Z gives stable elements • 1:1 up to 4020Ca • Ratio gradually rises (A>Z) unt ...
... • Stability is favoured by even numbers of protons and neutrons • Not usually equal numbers • Plotting neutron number (A) against proton number (Z) for all known nuclei, shows area of stability • For very light elements N ≈ Z gives stable elements • 1:1 up to 4020Ca • Ratio gradually rises (A>Z) unt ...
Nuclear Chemistry - Ector County ISD.
... first person to win two (the first, shared with her husband Pierre and Becquerel for discovering radioactivity; the second for discovering the radioactive elements radium and polonium). Radiation and nuclear reactions In 1902, Frederick Soddy proposed the theory that "radioactivity is the result of ...
... first person to win two (the first, shared with her husband Pierre and Becquerel for discovering radioactivity; the second for discovering the radioactive elements radium and polonium). Radiation and nuclear reactions In 1902, Frederick Soddy proposed the theory that "radioactivity is the result of ...
Alpha Beta Fission Fusion
... The decay reaction and T½ of a substance are specific to the isotope of the element undergoing radioactive decay. For example, Bi210 can undergo decay to Tl206 with a T½ of five days. Bi215, by comparison, undergoes decay to Po215 with a T½ of 7.6 minutes, and Bi208 undergoes yet another mode of ...
... The decay reaction and T½ of a substance are specific to the isotope of the element undergoing radioactive decay. For example, Bi210 can undergo decay to Tl206 with a T½ of five days. Bi215, by comparison, undergoes decay to Po215 with a T½ of 7.6 minutes, and Bi208 undergoes yet another mode of ...
Radioactivity Unit - hrsbstaff.ednet.ns.ca
... higher energy metastable (“sort-of-stable”) state, from which they decay some time later. During the decay, they emit photons of certain smaller frequencies, which we can then see, and the atoms return to their lower-energy stable state. This makes them glow in the dark. There are 3 types of radiati ...
... higher energy metastable (“sort-of-stable”) state, from which they decay some time later. During the decay, they emit photons of certain smaller frequencies, which we can then see, and the atoms return to their lower-energy stable state. This makes them glow in the dark. There are 3 types of radiati ...
Nurse Shark (Ginglymostoma cirratum)
... • the sum of that atom’s protons and neutrons • can only be found on the periodic table for stable atoms, cannot be found on the periodic table for an isotope ...
... • the sum of that atom’s protons and neutrons • can only be found on the periodic table for stable atoms, cannot be found on the periodic table for an isotope ...
Unit 3 – Atomic Structure
... energy • Beta decay – spontaneous decay of a nucleus that emits an electron and energy • Gamma decay – spontaneous decay of a nucleus that emits • Isotope -Atoms of the same element with different numbers of neutrons • Mass number -The total number of protons and neutrons in a nucleus • Subatomic pa ...
... energy • Beta decay – spontaneous decay of a nucleus that emits an electron and energy • Gamma decay – spontaneous decay of a nucleus that emits • Isotope -Atoms of the same element with different numbers of neutrons • Mass number -The total number of protons and neutrons in a nucleus • Subatomic pa ...
chp. 7
... ◦ Radioactivity is the process by which an unstable nucleus emits one or more particles or energy in the form of electromagnetic radiation ...
... ◦ Radioactivity is the process by which an unstable nucleus emits one or more particles or energy in the form of electromagnetic radiation ...
Adobe Acrobat file ()
... Quick problem: protons in your body What is the order of magnitude of the number of protons in your body? Of the number of neutrons? Of the number of electrons? Take your mass approximately equal to 70 kg. An iron nucleus (in hemoglobin) has a few more neutrons than protons, but in a typical water ...
... Quick problem: protons in your body What is the order of magnitude of the number of protons in your body? Of the number of neutrons? Of the number of electrons? Take your mass approximately equal to 70 kg. An iron nucleus (in hemoglobin) has a few more neutrons than protons, but in a typical water ...
Nuclear fission
In nuclear physics and nuclear chemistry, nuclear fission is either a nuclear reaction or a radioactive decay process in which the nucleus of an atom splits into smaller parts (lighter nuclei). The fission process often produces free neutrons and photons (in the form of gamma rays), and releases a very large amount of energy even by the energetic standards of radioactive decay.Nuclear fission of heavy elements was discovered on December 17, 1938 by German Otto Hahn and his assistant Fritz Strassmann, and explained theoretically in January 1939 by Lise Meitner and her nephew Otto Robert Frisch. Frisch named the process by analogy with biological fission of living cells. It is an exothermic reaction which can release large amounts of energy both as electromagnetic radiation and as kinetic energy of the fragments (heating the bulk material where fission takes place). In order for fission to produce energy, the total binding energy of the resulting elements must be less negative (higher energy) than that of the starting element.Fission is a form of nuclear transmutation because the resulting fragments are not the same element as the original atom. The two nuclei produced are most often of comparable but slightly different sizes, typically with a mass ratio of products of about 3 to 2, for common fissile isotopes. Most fissions are binary fissions (producing two charged fragments), but occasionally (2 to 4 times per 1000 events), three positively charged fragments are produced, in a ternary fission. The smallest of these fragments in ternary processes ranges in size from a proton to an argon nucleus.Apart from fission induced by a neutron, harnessed and exploited by humans, a natural form of spontaneous radioactive decay (not requiring a neutron) is also referred to as fission, and occurs especially in very high-mass-number isotopes. Spontaneous fission was discovered in 1940 by Flyorov, Petrzhak and Kurchatov in Moscow, when they decided to confirm that, without bombardment by neutrons, the fission rate of uranium was indeed negligible, as predicted by Niels Bohr; it wasn't.The unpredictable composition of the products (which vary in a broad probabilistic and somewhat chaotic manner) distinguishes fission from purely quantum-tunnelling processes such as proton emission, alpha decay and cluster decay, which give the same products each time. Nuclear fission produces energy for nuclear power and drives the explosion of nuclear weapons. Both uses are possible because certain substances called nuclear fuels undergo fission when struck by fission neutrons, and in turn emit neutrons when they break apart. This makes possible a self-sustaining nuclear chain reaction that releases energy at a controlled rate in a nuclear reactor or at a very rapid uncontrolled rate in a nuclear weapon.The amount of free energy contained in nuclear fuel is millions of times the amount of free energy contained in a similar mass of chemical fuel such as gasoline, making nuclear fission a very dense source of energy. The products of nuclear fission, however, are on average far more radioactive than the heavy elements which are normally fissioned as fuel, and remain so for significant amounts of time, giving rise to a nuclear waste problem. Concerns over nuclear waste accumulation and over the destructive potential of nuclear weapons may counterbalance the desirable qualities of fission as an energy source, and give rise to ongoing political debate over nuclear power.